Researchers Discover Use for Carbon Dioxide in Conversion of Biomass Into Biofuel
(PhysOrg.com) -- Researchers at Columbia University have successfully discovered a beneficial use for carbon dioxide in the conversion of organic materials, such as grass and bark, into fuel. Their findings show that if utilized on a broad scale, their technique could help significantly reduce overall carbon emissions, both from the use of carbon dioxide in biofuel production and the creation of a more energy-efficient production process. The study appears this week on the website of the Journal of Environmental Science & Technology.
Increasing global energy use coupled with the need to reduce greenhouse gas emissions such as carbon dioxide has resulted in the exploration of viable alternative fuel sources that are carbon neutral. Biomass fuels -- consisting of organic, biological materials—hold promise as renewable sources for energy, but present a double-edged sword: Current approaches for turning biomass into fuel involve a considerable amount of energy and water to form the steam needed to convert the raw, organic materials. In addition, the conventional conversion of such fuels typically leads to the emission of additional atmospheric carbon dioxide.
To solve this challenge, Marco Castaldi, assistant professor, and Heidi Butterman, postdoctoral researcher, in the department of earth and environmental engineering at Columbia’s Fu Foundation School of Engineering and Applied Science, have found that by using carbon dioxide in the actual conversion of biomass, the process becomes more energy efficient and reduces carbon dioxide emissions.
“Hopefully these findings will stimulate people to think about utilizing carbon dioxide and other waste streams to make chemicals and products that society wants,” said Castaldi, a chemical engineer. “This is what engineering does best, developing processes that can extract value from unwanted materials—to help make the world a better place.”
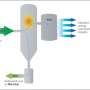
Gasification is a process that converts materials with high carbon content, such as coal, petroleum, or biomass, into volatile products—mostly hydrogen and carbon monoxide—by reacting the raw material at high temperatures with a controlled amount of oxygen and/or steam. The resulting mixture is a fuel called synthesis gas or syngas.
In their study, Castaldi, and Butterman, along with another postdoctoral researcher, Eilhann Kwon, processed 50 different kinds of biomass, including beach grass, pine needles, poplar wood, municipal solid waste and coal, from 25°C to 1000°C at rates of 1-100°C per minute in pure carbon dioxide and in a mixture of steam, nitrogen gas and carbon dioxide. They found that a carbon dioxide-steam mixture significantly increased the conversion of biomass to volatile products at lower temperatures.
When carbon dioxide and steam are present in gasification, the carbon dioxide reacts first to convert the solid fuel to syngas, leaving the steam to react with some of the syngas in a reaction called water-gas shift, which liberates some energy. The researchers found that by replacing 30 percent of the steam with carbon dioxide, the overall process is more efficient because the CO2 is more reactive than steam and can more readily access the carbon char (unprocessed biomass) for conversion into syngas.
The researchers believe that the use of carbon dioxide in biomass conversion, if applied on a mass scale, hold the potential to globally process tens to hundreds of megatons of carbon dioxide per year. According to their calculations, using CO2 during gasification of biomass fuels results in an additional emissions reduction than just the use of biofuels alone. For low-temperature gasification of beach grass, for example, the incorporation of carbon dioxide could create a beneficial use for 437 million metric tons of CO2 (based on estimated transportation fuel needs for 2008). For a typical automobile producing 6 metric tons of CO2/year this would be equivalent to removing 308 million vehicles from the road.
Carbon dioxide used in a mass-scale gasification process can be diverted from a variety of industrial sources, including power plant exhaust, future power plants that use syngas and compressed carbon dioxide, or from food and beverage manufactures that emit carbon dioxide as a by-product. Using industrial carbon dioxide would lead to a further reduction of emissions.
For Castaldi and Butterman, the next step in their research is to further develop and understand the mechanisms of this process, and look at different waste streams, such as municipal and agricultural waste, to investigate where catalysts can be incorporated to refine the resulting biofuel.
Provided by The Earth Institute at Columbia University (news : web)