Intestinal cells surprisingly active in pursuit of nutrition and defense
Every cell lining the small intestine bristles with thousands of tightly packed microvilli that project into the gut lumen, forming a brush border that absorbs nutrients and protects the body from intestinal bacteria. In the June 29, 2009 issue of the Journal of Cell Biology, Matthew McConnell, Matthew Tyska, and colleagues now find that microvilli extend their functional reach even further using a molecular motor to send vesicles packed with gut enzymes out into the lumen to get a head start on breaking down their substrates.
Microvilli have traditionally been viewed as passive scaffolds that increase the surface area of the gut wall. The apical plasma membrane tightly wraps around each protrusive bundle of actin, providing more space for nutrient processing and absorption. The motor protein myosin-1a (myo1a) maintains this structure by connecting the plasma membrane to the actin filaments.
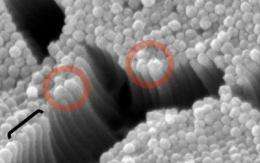
In 2007, Tyska and colleagues found that myo1a functions in isolated brush borders to actively move membrane along the length of the microvilli, like a "membrane escalator." To their surprise, at the top of these escalators—the tips of the microvilli—the membrane pinched off to form small vesicles that were released into the surrounding medium. According to Tyska, when they showed their data to gastroenterologists, they immediately asked "Why would brush borders do that? They're wasting perfectly good apical membrane!" Tyska therefore wanted to see if vesicle shedding was a bona fide physiological function for microvilli.
Sure enough, scanning electron micrographs of rat intestines showed protrusions at the tips of microvilli that looked similar to budding vesicles. And a look at the gut's contents revealed vesicles enriched in the brush border enzyme intestinal alkaline phosphatase (IAP). The vesicles were packed with classical brush border membrane proteins such as aminopeptidases and sugar-processing enzymes, suggesting that the vesicles were derived from microvilli. The vesicles also contained several proteins such as annexin A13 that bend cell membranes and could form part of the vesicle budding machinery.
One protein definitely involved in vesicle formation is myo1a. Myo1a knockout mice still produce lumenal vesicles but they are irregularly sized and no longer enriched in specific proteins like IAP. Tyska thinks that these knockout vesicles are actually chunks of microvillar membrane that are nonspecifically shed when myo1a isn't present to keep them attached to the actin core.
Returning to the gastroenterologists' question: Why would brush borders do that? McConnell et al. showed that the packaged enzymes were exposed on the vesicles' outer surface and were catalytically active. Releasing the enzymes in vesicles might increase their mixing with substrates in the gut's contents. Tyska is particularly interested in IAP, which has recently been shown to detoxify the bacterial outer-membrane component lipopolysaccharide. Releasing IAP in lumenal vesicles could be an important defense mechanism against intestinal pathogens.
More information: McConnell, R.E., et al. 2009. J. Cell Biol. doi:10.1083/jcb.200902147. www.jcb.org
Source: Rockefeller University (news : web)













