Frozen helium-4 may be an unusual 'superglass'
(PhysOrg.com) -- When helium is cooled to around 4 degrees above absolute zero, it turns liquid. Make it a couple of degrees cooler, and it becomes a "superfluid" that flows without resistance from its container, just as electrons flow without resistance in a superconductor.
Now pressurize the helium to about 50 atmospheres until it solidifies, and then cool it a lot more to about two-tenths of a degree above absolute zero, and it becomes -- well, there's a lot of argument about what it is. Perhaps a supersolid, or a solid with some superfluid moving through it.
In fact, it may be a superglass, report J.C. Séamus Davis, the James Gilbert White Distinguished Professor in the Physical Sciences at Cornell and colleagues in the May 1 issue of the journal Science.
The researchers are cautious in their conclusions, not insisting that the material is a glass but presenting evidence to support that idea, which was proposed by theorists about two years ago.
(All this refers to helium-4, the common variety you see in balloons. Helium-3, an isotope with two protons and only one neutron in its nucleus, has parallel but different properties. Cornell researchers Robert Richardson, David Lee and Douglas Osheroff earned a Nobel Prize for discovering the superfluid state of helium-3.)
In a solid, as scientists define it, atoms bond to one another in an orderly crystal lattice. In a liquid, the atoms freely move around. A glass is really a liquid flowing so slowly that it appears to be solid. Look out your window for a few hundred years, and you might notice it starting to sag.
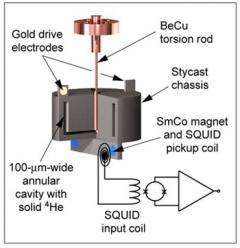
Although the theory that frozen helium might be a supersolid has been around for years, the first evidence that it was at least a super-something was provided in a 2004 experiment by Moses Chan at Penn State. Researchers there placed a tiny cylinder of frozen helium in a torsion oscillator, which rotates rapidly forward and back, like a washing machine agitator. The resonant frequency of the oscillator -- the one it naturally settles into -- depends on the mass it's trying to move around and back. The researchers found that below a critical temperature, some of the mass of the helium seemed to disappear.
Imagine holding an egg upright in your hand and twisting your wrist back and forth. There isn't much resistance because the inside is liquid and slides around. Hard-boil the egg and you feel all of its mass pushing back. Now suppose a hard-boiled egg decided not to push back: odd. That won't happen with eggs, but it does happen with solid helium near absolute zero.
One way to explain this is in terms of the famous Heisenberg uncertainty principle: We can't know both the position and velocity of a particle with great precision; the more we know about one, the less we know about the other. Near absolute zero atoms aren't moving very fast, so their position becomes very loose. The many atoms of helium overlap so much that they behave as a single atom -- a state of matter known as a Bose-Einstein condensate -- which is unaffected by anything around it and essentially frictionless.
Davis' group used an apparatus similar to Chan's, but "heated" the sample to a range of temperatures from near absolute zero up to about 300 milliKelvins (thousandths of a degree above absolute zero). After each heating they watched how the resonant frequency of the oscillator changed over a period of many hours, essentially measuring how long it took for the material to "refreeze." What they found was consistent with a material that becomes more and more glass-like as the temperature increases, rather than something that responds like a crystalline solid.
Davis likens the effect to a glass-blowing demonstration. At room temperature glass seems pretty solid. At around 1,500 degrees Celsius it begins to melt and flow at speeds visible to humans. As the temperature rises it flows more freely. The dependence of these ultraslow flow properties on temperature can be used to distinguish a glass from a crystal, Davis explained. He compares heating glass to heating a diamond, which remains an ordered crystal no matter how hot it gets, until it reaches its melting point (3550 degrees Celsius). Below the melting point no slow changes would be seen over time after heating.
The second surprise was that only when the glasslike properties freeze out does the frictionless superflow signal discovered by Chan appear. "This is interesting not just because it appears glass-like and not just because it shows the Chan signal but because it shows a relationship between the two," Davis said. "We think that when this glass freezes, the superfluid begins to move, and such a state could be called a superglass."
Provided by Cornell University (news : web)