Rice researchers unzip the future
Scientists at Rice University have found a simple way to create basic elements for aircraft, flat-screen TVs, electronics and other products that incorporate sheets of tough, electrically conductive material.
And the process begins with a zipper.
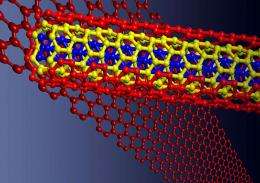
Research by the Rice University lab of Professor James Tour, featured on the cover of the April 16 issue of the journal Nature, has uncovered a room-temperature chemical process that splits, or unzips, carbon nanotubes to make flat nanoribbons. The technique makes it possible to produce the ultrathin ribbons in bulk quantities.
These ribbons are straight-edged sheets of graphene, the single-layer form of common graphite found in pencils. You'd have to place thousands of them side by side to equal the width of a human hair, but tests show graphene is 200 times stronger than steel.
"If you want to make conductive film, this is what you want," said Tour, Rice's Chao Professor of Chemistry and also a professor of mechanical engineering and materials science and computer science. "As soon as we started talking about this process, we began getting calls from manufacturers that recognized the potential."
The process involves sulfuric acid and potassium permanganate, which have been in common use since the 1890s. This chemical one-two punch attacks single and multiwalled carbon nanotubes, reacting with the carbon framework and unzipping them in a straight line.
The unzipping action can start on the end or in the middle, but the result is the same - the tubes turn into flat, straight-edged, water-soluble ribbons of graphene. When produced in bulk, these microscopic sheets can be "painted" onto a surface or combined with a polymer to let it conduct electricity.
Nanotubes have been used for that purpose already. "But when you stack two cylinders, the area that is touching is very small," Tour said. "If you stack these ribbons into sheets, you have very large areas of overlap. As an additive for materials, it's going to be very large, especially for conductive materials."
He credited Rice postdoctoral research associate Dmitry Kosynkin with the discovery. Kosynkin is lead author of the Nature paper, with contributions from graduate students Amanda Higginbotham, Jay Lomeda and B. Katherine Price, postdoctoral researcher Alexander Sinitskii, visiting scientist Ayrat Dimiev and Tour.
Kosynkin made the find while studying oxidation processes involving nanotubes. "Dmitry came to me and said he had nanoribbons," recalled Tour. "It took a while to convince me, but as soon as I saw them I realized this was huge."
Nearly all of the nanotubes subjected to unzipping turn into graphene ribbons, Tour said, and the basic process is the same for single or multiwalled tubes. Single-walled carbon nanotubes convert to sheets at room temperature and are good for small electronic devices because the width of the unzipped sheet is highly controllable. But the multiwalled nanotubes are much cheaper starting materials, and the resulting nanoribbons would be useful in a host of applications.
That's why Tour is banking on bulk, made possible by processing multiwalled tubes, which unzip in one hour at 130 to 158 degrees Fahrenheit. (Until now, making such material in more than microscopic quantities has involved a chemical vapor deposition process at more than 1,500 F.) "Multiwalled carbon nanotubes are concentric tubes, like Russian nesting dolls," he said. "We cut through 20 walls, one at a time, during the reaction process."
At first, the process of isolating nanoribbons "involved a lot of excruciating washing," he said. "But we've found a much easier way, which we needed to do to get industry to start taking it from here.
"If a company wants to produce these, they could probably start selling small quantities within six months. To scale it up and sell ton quantities, it might take a couple of years. That's just a matter of having the right reactors. But the chemistry is all there. It's very simple."
Tour is excited by the possibility that conductive nanoribbons could replace indium tin oxide (ITO), a material commonly used in flat-panel displays, touch panels, electronic ink and solar cells. "ITO is very expensive, so lots of people are looking for substitutes that will give them transparency with conductivity," he said.
"People have made thin films of nanotubes that fit the bill, but I think this will enable even thinner films, with the equivalent conductivity or better."
He envisioned nanoribbon-coated paper that could become a flexible electronic display, and he's already experimenting with nanoribbon-infused ink for ink-jet printers. "We're printing transistors and radio-frequency identification tags, printing electronics with these inks," he said.
"This is going to be the new material for many applications."
Tour said discussions are already underway with several companies looking into large-scale production of nanoribbons and with others interested in specific applications for nanoribbons in their core product technologies. Formal industrial partnering has already begun through Rice's Office of Technology Transfer.
Source: Rice University (news : web)