New Nanoparticle to Help Researchers Study Angiogenesis
(PhysOrg.com) -- Adah Almutairi, Ph.D., assistant professor in the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego, is first author of a paper recently published in the Proceedings of the National Academy of Sciences (PNAS.) The work of Almutairi and her former colleagues at UC Berkeley, along with researchers from the Washington University School of Medicine, describes a novel synthetic nanoparticle developed for noninvasive imaging of angiogenesis.
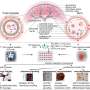
Angiogenesis, or the formation of new blood vessels, plays an important role in many human diseases such as cancer or heart disease such as weakening of the heart muscle (cardiomyopathy) or thickening and hardening of the arteries (atherosclerosis). Nanotechnology has the potential to revolutionize the diagnosis and treatment of these disorders, and the research team has developed a biodegradable nanoprobe to target a biological marker known to modulate angiogenesis.
“One challenge of nanoparticles has been the difficulty in targeting where they go, because of the properties of size and structure,” said Almutairi. “Either they are unable to diffuse into tissue, because the nanoparticles are too large, or - if too small - they clear out of the system too rapidly.” The nanoparticles also have to be structurally camouflaged so they aren’t attacked by the system’s immune system.
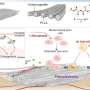
The researchers designed a nanoprobe that is commercially viable because it is biodegradable and dissolves so has no long-term, toxic effect, according to Almutairi.
“This particle is small enough to easily circulate - about ten to 12 nanometers in size, where most nanoparticles are about 50 nanometers,” Almutairi said. “We also ‘decorated’ it with targeting groups in a novel way so that it can recognize diseased tissue.”
Most importantly, this nanoprobe has increased selectivity for cells that express a specific integrin receptor, αvβ3, which serves as a biological marker for angiogenesis. These adhesive receptors are critical for the proliferation, survival and function of new blood vessels.
Almutairi says she hopes to collaborate with cancer researchers who can use this nanoparticle in the development of new diagnostics and therapeutics.
Provided by University of California, San Diego