This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Enhancing energy conversion: Pt-Co@NCS catalyst demonstrates synergy for enhanced alkaline hydrogen evolution
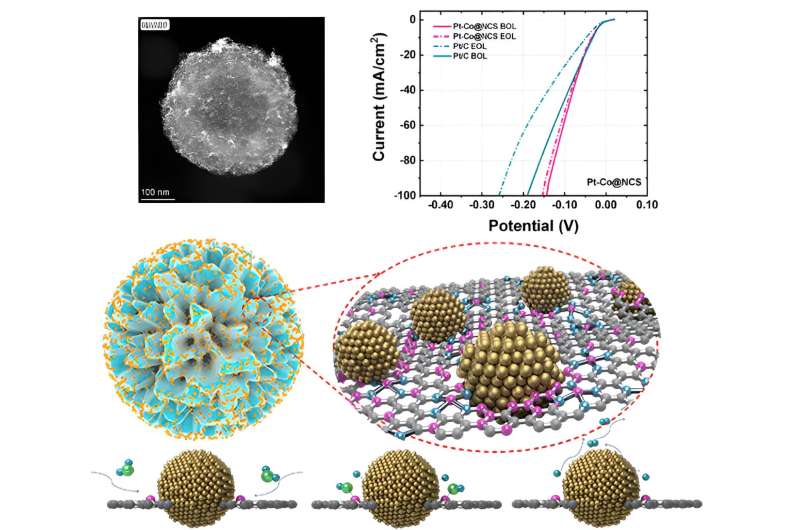
A study published in the journal Materials Futures introduces the Pt-Co@NCS catalyst, showcasing a remarkable synergy between Pt nanoparticles and Co single atoms on a nitrogen-doped carbon scaffold. This innovative design overcomes the hurdle of slow water dissociation, leading to exceptional performance in alkaline hydrogen evolution reaction (HER).
With a current density of 162.8 mA cm-2, a Tafel slope of 26.2 mV dec-1, and a superior mass activity of 15.75 mA μg Pt-1, the Pt-Co@NCS catalyst marks a significant leap forward in enhancing hydrogen evolution efficiency. These findings promise a bright future for sustainable energy solutions.
In recent years, innovative catalysts have been engineered to enhance the local environment at the Pt-group interface on traditional oxide supports such as CeO2, TiO2, and SiO2. However, these oxide supports are inferior to carbon supports due to limited charge transfer and additional energy losses from internal resistance at high current densities.
The need for improved water dissociation kinetics has led to the use of nanostructured, nitrogen-doped carbon supports. This approach has demonstrated a synergistic interaction with water at the defective nitrogen-doped sites, significantly enhancing HER performance.
The study reported the successful synthesis of an innovative Pt-Co@NCS catalyst, overcoming the traditional bottleneck of slow water dissociation.
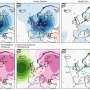
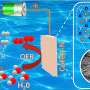
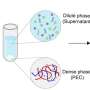
In this paper, the authors constructed a hierarchically porous N-doped carbon scaffold (NCS) catalyst containing Co single atoms and Pt nanoparticles for alkaline HER. The porous structure was created through the polymerization of m-phenylenediamine and etching of a silicon oxide coating. Pt and Co precursors were then pyrolyzed in the confined space of NCS, transforming into tiny Pt nanoparticles and atomically dispersed Co-N4 sites.
The key to its remarkable performance lies in the unique porous concave structure paired with a nitrogen-rich defective surface, which collectively enhances hydrophilicity and catalytic interaction around the Pt sites. This unique structure also minimized the potential aggregation of metal species, enhancing both the long-term activity and stability of Pt-Co@NCS.
The synergistic interaction of Pt nanoparticles and Co single atoms, along with the nanoscale geometrical effects, significantly enhances water dissociation by promoting OH- adsorption to the Co single atoms in an alkaline environment. Rigorous CO stripping and deuterium labeling experiments confirmed the promoted water dissociation pathway related to these synergistic interactions. This research highlights the essential role of tailoring the microenvironment around catalytic sites, especially for alkaline applications, to enhance activity and stability.
Based on the demonstrated effectiveness of the Pt-Co@NCS catalyst in enhancing HER efficiency in alkaline media, the future of material development for single-atom and crystalline synergistic catalysis appears highly promising. This field is expected to further explore the vast potential of high-density atomically dispersed metal catalysts on various materials.
Future research could focus on diversifying the types of metal atoms and doping elements in the substrate to tailor catalytic properties for specific reactions beyond HER, as well as exploring the long-term stability and scalability of these catalysts. Moreover, developing in situ and real-time techniques for monitoring synergistic catalytic processes at the atomic level is crucial for understanding the dynamic interactions within the catalyst microenvironment.
Through these efforts, the catalysis community is likely to make significant strides in creating more efficient, durable, and economically viable catalysts to support sustainable energy technologies.
More information: Chengyong Shu et al, Synergistic Effect between Co Single Atoms and Pt Nanoparticles for Efficient Alkaline Hydrogen Evolution, Materials Futures (2024). DOI: 10.1088/2752-5724/ad521f
Provided by Songshan Lake Materials Laboratory