This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
One essential step for a germ cell, one giant leap for the future of reproductive medicine
Although assisted reproductive technologies (ARTs), such as in vitro fertilization (IVF), have had a tremendous impact on treating certain forms of infertility—not all forms of infertility can be targeted with existing strategies.
Recently, one powerful technology has emerged—known as human in vitro gametogenesis (IVG)—using pluripotent stem cells (PSCs), such as induced pluripotent stem cells (iPSCs) from patients, to generate human germ cells with the capacity to potentially give rise to mature gametes in culture, offering a gateway to treating all form of infertility—independent of gender.
Nevertheless, human IVG research still remains in its infancy, with the current goal being to reconstitute the complete process of human gametogenesis. To date, one major challenge has been to recapitulate in the founder population of germ cells, or the human primordial germ cells (hPGCs), a hallmark event known as epigenetic reprogramming —in which the inherited parental "memory" of cells, present on its DNA, is reset/erased— that is required for proper germ cell differentiation.
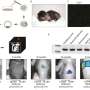
Now, in a study published in Nature, researchers at the Institute for the Advanced Study of Human Biology (WPI-ASHBi) in Kyoto University, led by Dr. Mitinori Saitou, have identified robust culture conditions necessary to drive epigenetic reprogramming and germ cell differentiation into precursors of mature gametes, the mitotic pro-spermatogonia and pro-oogonia with the capacity for extensive amplification, achieving a new milestone for human IVG research.
Previous work by Saitou's team and other groups were successful in generating so-called human primordial germ cell-like cells (hPGCLCs) from PSCs in vitro, which recapitulated several fundamental features of hPGC, including the capacity to propagate. However, these hPGCLCs were unable to undergo epigenetic reprogramming and differentiation.
Although such limitations could be bypassed by aggregating hPGCLCs with mouse embryonic (non-germinal) gonadal cells to mimic the microenvironment of the testis/ovary, this process is highly inefficient (with approximately only 1/10th of cells differentiating). Furthermore, the introduction of non-human cells is neither ideal nor practical from a clinical application perspective. Therefore, in order to achieve the ultimate goal of human IVG research, it is essential to identify the minimal culture conditions necessary to generate mature human gametes.
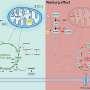
In their new study, Saitou and colleagues conducted a cell culture-based screen to identify potential signaling molecules required to drive epigenetic reprogramming and differentiation of hPGCLCs into mitotic pro-spermatogonia and oogonia. Surprisingly, the authors found that the well-established developmental signaling molecule, bone morphogenetic protein (BMP), played a crucial role in this reprogramming and differentiation process of hPGCLCs.
"Indeed, considering that BMP signaling already has an established role in germ cell specification, it was highly unexpected that it also drives hPGCLC epigenetic reprogramming," says Saitou.
These hPGCLC-derived mitotic pro-spermatogonia/oogonia not only displayed similarities in gene expression and epigenetic profiles to that of actual hPGC differentiation in our bodies, but also underwent extensive amplification (over 10 billion-fold).
"Our approach enables near-indefinite amplification of mitotic pro-spermatogonia and oogonia in culture and we now also have the ability to store and re-expand these cells as needed," says Saitou.
The authors also reveal the potential mechanisms of how BMP signaling may be leading to epigenetic reprogramming and hPGCLC differentiation.
"BMP (signaling) appears to be attenuating the MAPK/ERK (mitogen-activated protein kinase/extracellular-regulated kinase) signaling pathway and both the de novo and maintenance activities of DNMT (DNA methyltransferase), but further investigation will be necessary to determine the precise mechanism and whether this is direct or indirect," explains Saitou.
"Our study represents not only a fundamental advance in our understanding of human biology and the principles behind epigenetic reprogramming in humans but also a true milestone in human IVG research" says Saitou.
Saitou says, "Although many challenges remain and the path will certainly be long, especially when considering the ethical, legal, and social implications associated with the clinical application of human IVG, nevertheless, we have now made one significant leap forward towards the potential translation of IVG into reproductive medicine."
More information: Yusuke Murase et al, In vitro reconstitution of epigenetic reprogramming in the human germ line, Nature (2024). DOI: 10.1038/s41586-024-07526-6
Journal information: Nature
Provided by Kyoto University