March 21, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
An approach to design high-power lithium sulfur batteries
Lithium–sulfur (Li–S) batteries are a promising alternative to lithium–ion batteries (LiBs), the most common rechargeable battery technology. As sulfur is abundant on Earth, these batteries could be cheaper and more environmentally friendly than LiBs, while also potentially exhibiting higher energy densities.
Despite these advantages, the deployment of Li–S batteries has so far been limited, as many of these batteries also have a low cycle life and a high self-discharge rate. In addition, the predicted high energy density of Li–S batteries often becomes far lower when in real applications, due to the high rates at which they charge and discharge.
A chemical reaction that plays a central role in ensuring the high capacity of Li–S batteries is the so-called sulfur reduction reaction (SRR). This reaction has been widely studied, yet its kinetic tendencies at high current rates remain poorly understood.
Researchers at the University of Adelaide, Tianjin University and Australian Synchrotron recently carried out a study aimed at delineating the kinetic trend of SRR, to inform the future development of high-power Li–S batteries. Their paper, published in Nature Nanotechnology, also introduces a nanocomposite carbon electrocatalyst that was found to boost the performance of Li–S batteries, attaining a discharge capacity retention of approximately 75%.
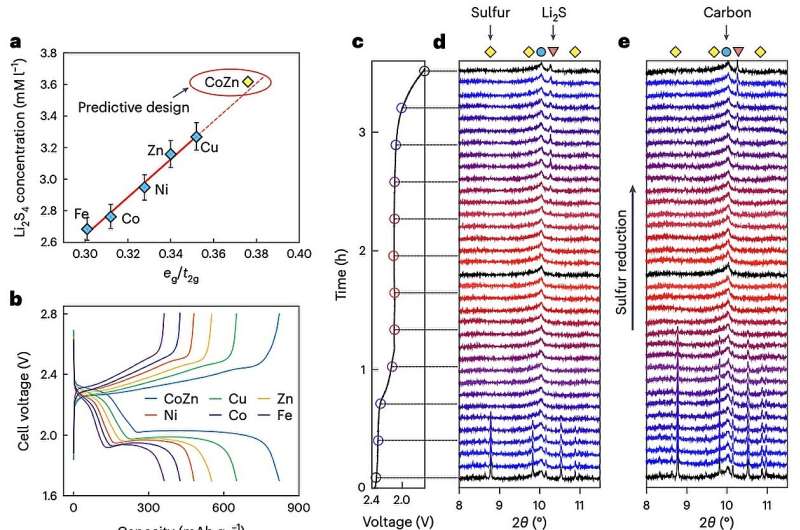
"The activity of electrocatalysts for the sulfur reduction reaction (SRR) can be represented using volcano plots, which describe specific thermodynamic trends," Huan Li, Rongwei Meng and their colleagues wrote in their paper. "However, a kinetic trend that describes the SRR at high current rates is not yet available, limiting our understanding of kinetics variations and hindering the development of high-power Li||S batteries. Using Le Chatelier's principle as a guideline, we establish an SRR kinetic trend that correlates polysulfide concentrations with kinetic currents."
To further examine the kinetic trend of the SRR at high currents, the researchers also collected synchrotron X-ray adsorption spectroscopy measurements and ran various molecular orbital computations. Overall, their results suggest that orbital occupancy in catalysts based on transition metals is linked to the concentration of polysulfide in batteries, and consequently also SRR kinetic predictions.
Based on the kinetic trend they delineated, Li, Meng and their collaborators designed a new nanocomposite electrocatalyst comprised of a carbon-based material and CoZn clusters. They then integrated this catalyst in a Li–S battery cell and tested its performance, focusing on its charge-discharge rates.
"When the electrocatalyst is used in a sulfur-based positive electrode (5 mg cm−2 of S loading), the corresponding Li||S coin cell (with an electrolyte:S mass ratio of 4.8) can be cycled for 1,000 cycles at 8°C (that is, 13.4 A gS−1, based on the mass of sulfur) and 25°C," the researchers wrote.
"This cell demonstrates a discharge capacity retention of about 75% (final discharge capacity of 500 mAh gS−1) corresponding to an initial specific power of 26,120 W kgS−1 and specific energy of 1,306 Wh kgS−1."
Overall, the recent study by Li, Meng and their colleagues shows that increased polysulfide concentrations promote faster SRR kinetics; thus, catalysts that boost polysulfide concentration could speed up this reaction. This result was validated both via theoretical computations and experimental measurements.
Building on their observations, the researchers already introduced one electrocatalyst that was found to enhance the capacity retention and cyclic stability of an Li–S battery. In the future, their work could inspire the design of other promising catalysts, potentially contributing to the development of new high-power Li–S battery technologies.
More information: Huan Li et al, Developing high-power Li||S batteries via transition metal/carbon nanocomposite electrocatalyst engineering, Nature Nanotechnology (2024). DOI: 10.1038/s41565-024-01614-4
Journal information: Nature Nanotechnology
© 2024 Science X Network