Quantum chemical computations help screen better cathode materials for high Li–S batteries
Energy storage systems with high energy density are essential to fulfilling the ever-increasing demands of electronic devices, electric vehicles, and smart grids for intermittent solar or wind power. The Lithium-sulfur (Li-S) battery is a promising candidate for next-generation energy storage, with an extremely high theoretical energy density that is five to seven times higher than that of conventional LIBs.
However, a number of obstacles hinder practical applications of Li–S batteries. One of the major issues is the diffusion of polysulfide intermediates from the cathode, which causes the irreversible loss of active materials and capacity decay. Nanocarbon with a nonpolar surface as cathode materials cannot provide sufficient binding and confining effects to maintain polysulfides within the cathode. Moreover, the poor electrochemical contact caused by the weak combination between active polysulfides and nanocarbon also impedes the rapid and steady cycling of Li–S cells.
"Heteroatom doping is believed to be a promising route for the adsorption and immobilization of polysulfide intermediates," says Prof. Qiang Zhang, a researcher from Tsinghua University, China. "However, the origin of the anchoring effect afforded by heteroatoms is still not clear, which largely limits the improvement of polysulfides adsorption and the rational design of cathode materials."
Most recently, Prof. Q. Zhang and co-workers from Tsinghua University together with Prof. B. Li from Institute of Metal Research reported a theoretical study on the ability of a series of doped nanocarbon materials to trap polysulfides. It showed that by forming a 'lithium bond' (an analogue of 'H bond'), the chemical modification using N or O dopant significantly enhances the interaction between the carbon host and the polysulfide guests and thereby effectively prevents shuttle of polysulfides.
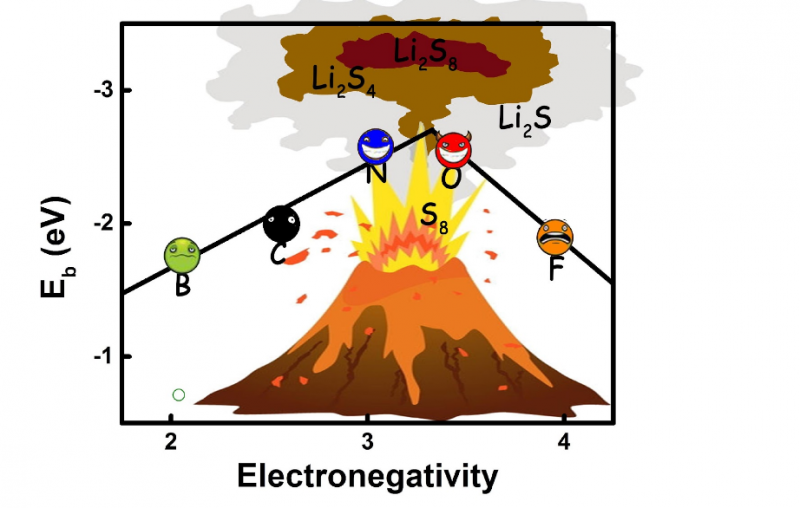
"For the first time, we conducted a parallel quantum chemical screening process to pick the most effective doping elements that helps confine the polysulfides." Tingzheng Hou, the first author says. "It turned out that the N and O doping in nanocarbon materials can form a strong dipole-dipole electrostatic interaction, which was for the first time identified as the dominant interaction between doped nanocarbon and lithium polysulfides, while F, B, P, S and Cl dopants were incapable of forming that."
Experimental work reported from other researchers accorded with this predictive result. For instance, the N-doped graphene paper electrode exhibited a high specific capacity of approximately 1000 mAh g-1 after 100 cycles and excellent coulombic efficiency of 98 percent for catholyte-type Li-S cell. A much-extended life of over 2000 cycles and an extremely low capacity-decay rate of 0.028 percent per cycle can thus be achieved.
"To achieve the strong-couple effect towards polysulfides, we proposed a set of rules for the rational design of doped carbon scaffolds in Li–S batteries based on our calculation," says Hou, "With these conditions fulfilled, the doped carbon could offer a strong dipole with lone pair election to form a strong electrostatic dipole-dipole interaction with polysulfides and enhance the interaction. The key factor is the electronegativity of the doping atoms."
To elucidate the importance of electronegativity, an implicit volcano plot relationship correlating the electronegativity of doping atoms to the adsorption energies was proposed by Qiang and coworkers to shed light on the formation of the strong anchoring effect. This relationship affords new understanding of the screening and rational design of doped nanocarbon materials for immobilizing polysulfides.
"If we go step further from the rules and the volcano plot to seek a breakthrough beyond the maximum limit of mono-doping, there are co-doping nanomaterials in which two or more dopants adjacent to each other synergistically strengthen the dipole moment and offer even better affinity to polysulfides." said Prof. Qiang. In the near future, they will further study the synergy effect of co-doping and explore the possibility of further enhancing the interfacial interactions in the cathode interface.
More information: Ting-Zheng Hou et al. Design Principles for Heteroatom-Doped Nanocarbon to Achieve Strong Anchoring of Polysulfides for Lithium-Sulfur Batteries, Small (2016). DOI: 10.1002/smll.201600809
Journal information: Small
Provided by Tsinghua University