Less is more when it comes to soot
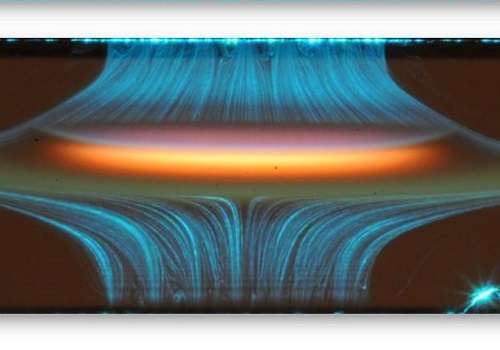
Small particles emitted into air during the burning of hydrocarbon fuels damage the human respiratory system and enhance the greenhouse effect. In their agglomerated form, these particles form soot that consists predominantly of highly condensed carbon atoms. The formation of soot is widely studied, but models are often inaccurate because the amount and size of the carbon particles depends on many factors, including the fuel used and the combustion conditions.
"These have often been neglected in previous studies," said Hong Im from the KAUST Clean Combustion Research Center (CCRC). He noted that consideration of these molecules is essential for accurate prediction of soot levels.
The researchers improved the way to predict the formation of soot precursors in the flames, or the so-called polycyclic aromatic hydrocarbons (PAH).
PAHs are organic molecules containing rings of carbon atoms with surrounding hydrogen atoms. They form during the burning process when smaller molecules collide and combine into larger ones. The molecules themselves cluster and under some conditions form carbon soot particles. A detailed understanding of the formation pathways for PAH is therefore essential to reduce soot formation and to improve the efficiency of the burn process.
Because of their larger number of atoms, previous computer models have been limited by the size of the PAH considered in the calculations. In their work, the researchers have identified more accurate chemical pathways to larger PAHs by carefully examining their complex reaction mechanisms.
The model developed in collaboration with the National University of Ireland, Galway and Saudi Aramco describes reaction pathways that lead to the formation of PAH with up to seven rings of carbons—24 carbon atoms in total.
Deploying the model, the research shows that accurate predictions must not only take PAH molecules into account, but that the larger PAH molecules are those that contribute most to the production of soot.
Although current computations are based on a simple fuel, future work will extend other fuel types such as gasoline or jet fuels. In addition, Im explained, dynamic effects are also important.
"Studies of soot formation under the influence of turbulent fluctuations are currently being investigated," Im said.
More information: Prabhu Selvaraj et al. A computational study of ethylene–air sooting flames: Effects of large polycyclic aromatic hydrocarbons, Combustion and Flame (2016). DOI: 10.1016/j.combustflame.2015.10.017
Provided by KAUST