Quantum effects at work in the world's smelliest superconductor
The quantum behaviour of hydrogen affects the structural properties of hydrogen-rich compounds, which are possible candidates for the elusive room temperature superconductor, according to new research co-authored at the University of Cambridge.
New theoretical results, published online in the journal Nature, suggest that the quantum nature of hydrogen - meaning that it can behave like a particle or a wave - strongly affects the recently discovered hydrogen sulphur superconductor, a compound that when subjected to extremely high pressure, is the highest-temperature superconductor yet identified. This new step towards understanding the underlying physics of high temperature superconductivity may aid in the search for a room temperature superconductor, which could be used for applications such as levitating trains, lossless electrical grids and next-generation supercomputers.
Superconductors are materials that carry electrical current with zero electrical resistance. Low-temperature, or conventional, superconductors were first identified in the early 20th century, but they need to be cooled close to absolute zero (zero degrees on the Kelvin scale, or -273 degrees Celsius) before they start to display superconductivity. For the past century, researchers have been searching for materials that behave as superconductors at higher temperatures, which would make them more suitable for practical applications. The ultimate goal is to identify a material which behaves as a superconductor at room temperature.
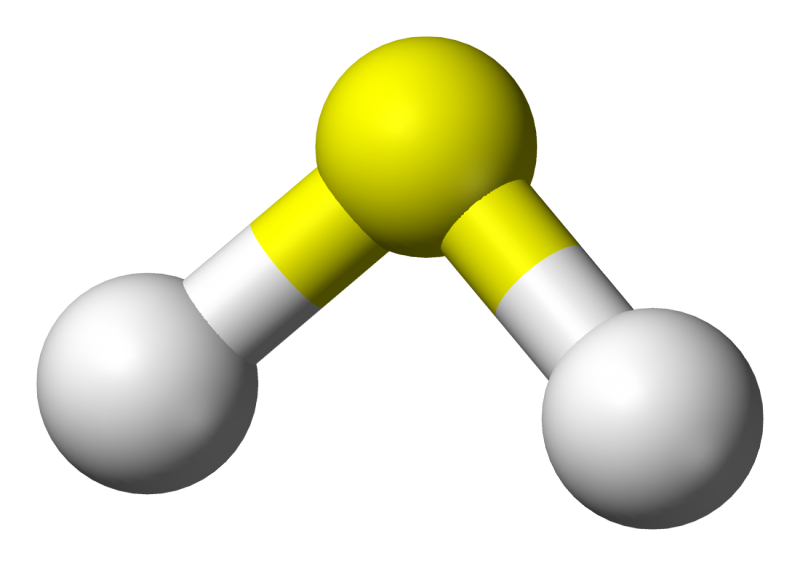
Last year, German researchers identified the highest temperature superconductor yet - hydrogen sulphide, the same compound that gives rotten eggs their distinctive odour. When subjected to extreme pressure - about one million times higher than the Earth's atmospheric pressure - this stinky compound displays superconducting behaviour at temperatures as high as 203 Kelvin (-70 degrees Celsius), which is far higher than any other high temperature superconductor yet discovered.
Since this discovery, researchers have attempted to understand what it is about hydrogen sulphide that makes it capable of superconducting at such high temperatures. Now, new theoretical results suggest that the quantum behaviour of hydrogen may be the reason, as it changes the structure of the chemical bonds between atoms. The results were obtained by an international collaboration of researchers led by the University of the Basque Country and the Donostia International Physics Center, and including researchers from the University of Cambridge.
The behaviour of objects in our daily life is governed by classical, or Newtonian, physics. If an object is moving, we can measure both its position and momentum, to determine where an object is going and how long it will take to get there. The two properties are inherently linked.
However, in the strange world of quantum physics, things are different. According to a rule known as Heisenberg's uncertainty principle, in any situation in which a particle has two linked properties, only one can be measured and the other must be uncertain.
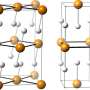
Hydrogen, being the lightest element of the periodic table, is the atom most strongly subjected to quantum behaviour. Its quantum nature affects structural and physical properties of many hydrogen compounds. An example is high-pressure ice, where quantum fluctuations of the proton lead to a change in the way that the molecules are held together, so that the chemical bonds between atoms become symmetrical.
The researchers behind the current study believe that a similar quantum hydrogen-bond symmetrisation occurs in the hydrogen sulphide superconductor.
Theoretical models that treat hydrogen atoms as classical particles predict that at extremely high pressures - even higher than those used by the German researchers for their record-breaking superconductor - the atoms sit exactly halfway between two sulphur atoms, making a fully symmetrical structure. However, at lower pressures, hydrogen atoms move to an off-centre position, forming one shorter and one longer bond.
The researchers have found that when considering the hydrogen atoms as quantum particles behaving like waves, they form symmetrical bonds at much lower pressures - around the same as those used for the German-led experiment, meaning that quantum physics, and symmetrical hydrogen bonds, were behind the record-breaking superconductivity.
"That we are able to make quantitative predictions with such a good agreement with the experiments is exciting and means that computation can be confidently used to accelerate the discovery of high temperature superconductors," said study co-author Professor Chris Pickard of Cambridge's Department of Materials Science & Metallurgy.
According to the researcher's calculations, the quantum symmetrisation of the hydrogen bond has a tremendous impact on the vibrational and superconducting properties of hydrogen sulphide. "In order to theoretically reproduce the observed pressure dependence of the superconducting critical temperature the quantum symmetrisation needs to be taken into account," said the study's first author, Ion Errea, from the University of the Basque Country and Donostia International Physics Center.
The discovery of such a high temperature superconductor suggests that room temperature superconductivity might be possible in other hydrogen-rich compounds. The current theoretical study shows that in all these compounds, the quantum motion of hydrogen can strongly affect the structural properties, even modifying the chemical bonding, and the electron-phonon interaction that drives the superconducting transition.
"Theory and computation have played an important role in the hunt for superconducting hydrides under extreme compression," said Pickard. "The challenges for the future are twofold - increasing the temperature towards room temperature, but, more importantly, dramatically reducing the pressures required."
More information: Ion Errea et al. Quantum hydrogen-bond symmetrization in the superconducting hydrogen sulfide system, Nature (2016). DOI: 10.1038/nature17175
Journal information: Nature
Provided by University of Cambridge