February 8, 2016 report
A new way to make higher quality bilayer graphene
(Phys.org)—A team of researchers with members from institutions in the U.S., Korea and China has developed a new way to make bilayer graphene that is higher in quality than that produced through any other known process. In their paper published in Nature Nanotechnology, the team describes the technique they developed and the possible uses for the bilayer graphene that is produced.
Graphene is, of course, a flat material made from just single carbon atoms; it forms in a honeycomb pattern and has been found to have excellent electrical properties—one hindrance to using graphene in many applications has been the lack of a bandgap. That hindrance was partially overcome back in 2009 when a team working in the U.S. found that creating two layers of graphene bonded together and then applying electricity could cause a bandgap to occur. Since that time, researchers have been looking for ways to create such bilayer graphene in a way that could be commercialized. In this latest effort, the researchers report on a new technique they have developed that they claim produces the highest quality bilayer graphene yet.
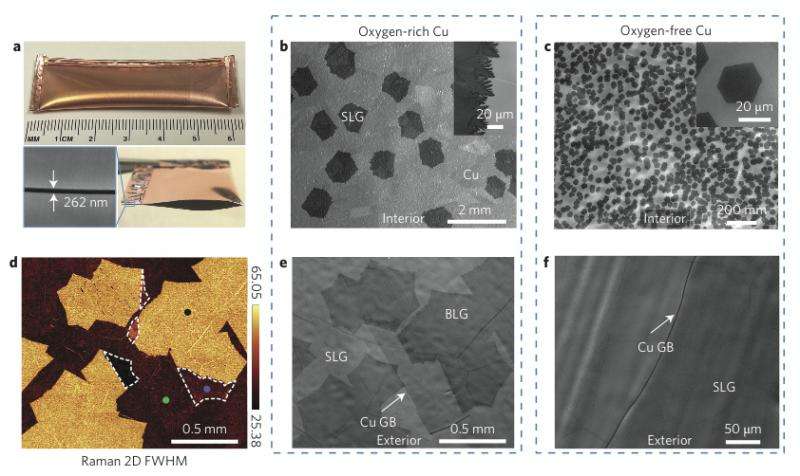
The current method used to create graphene is to use chemical vapor deposition—in creating bilayer graphene, researchers have to make sure that the two layers are in proper alignment with one another, outcomes are typically labeled mis-oriented or of a Bernal-type—the latter is obviously the outcome desired with the result called an AB-stacked configuration. The new technique developed by the team results in just such a configuration, it involves allowing a small amount of oxygen to enter a vapor deposition chamber where the graphene is being grown—the oxygen combines with a copper foil substrate causing a disassociation between methane molecules, releasing carbon atoms which then diffuse through the foil causing a second layer of graphene to form.
The team reports that the electrical properties of the resultant material is comparable to graphene produced from graphite, and it has a band gap of approximately 125meV, which the team acknowledges is not high enough for making transistors—but, they note it would likely work very well in optical applications such as for creating tunable photoemitters and/or detectors.
More information: Yufeng Hao et al. Oxygen-activated growth and bandgap tunability of large single-crystal bilayer graphene, Nature Nanotechnology (2016). DOI: 10.1038/nnano.2015.322
Abstract
Bernal (AB)-stacked bilayer graphene (BLG) is a semiconductor whose bandgap can be tuned by a transverse electric field, making it a unique material for a number of electronic and photonic devices. A scalable approach to synthesize high-quality BLG is therefore critical, which requires minimal crystalline defects in both graphene layers4, 5 and maximal area of Bernal stacking, which is necessary for bandgap tunability6. Here we demonstrate that in an oxygen-activated chemical vapour deposition (CVD) process, half-millimetre size, Bernal-stacked BLG single crystals can be synthesized on Cu. Besides the traditional 'surface-limited' growth mechanism for SLG (1st layer), we discovered new microscopic steps governing the growth of the 2nd graphene layer below the 1st layer as the diffusion of carbon atoms through the Cu bulk after complete dehydrogenation of hydrocarbon molecules on the Cu surface, which does not occur in the absence of oxygen. Moreover, we found that the efficient diffusion of the carbon atoms present at the interface between Cu and the 1st graphene layer further facilitates growth of large domains of the 2nd layer. The CVD BLG has superior electrical quality, with a device on/off ratio greater than 104, and a tunable bandgap up to ∼100 meV at a displacement field of 0.9 V nm−1.
Journal information: Nature Nanotechnology
© 2016 Phys.org