October 19, 2015 report
Scientists experimentally demonstrate 140-year-old prediction: A gas in perpetual non-equilibrium
(Phys.org)—In 1876, the Austrian physicist Ludwig Boltzmann noticed something surprising about his equations that describe the flow of heat in a gas. Usually, the colliding gas particles eventually reach a state of thermal equilibrium, the point at which no net flow of heat energy occurs. But Boltzmann realized that his equations also predict that, when gases are confined in a specific way, they should remain in persistent non-equilibrium, meaning a small amount of heat is always flowing within the system.
Now for the first time, physicists at JILA, the National Institute of Standards and Technology, and the University of Colorado at Boulder have experimentally realized a three-dimensional cloud of gas that never reaches thermal equilibrium, just as Boltzmann predicted nearly 140 years ago. The work builds on research from 2002, in which a persistent non-equilibrium state was observed in a two-dimensional gas.
"It's a long-delayed realization, call it a vindication, of one of the great Boltzmann's many interesting ideas," coauthor Eric Cornell at JILA told Phys.org. "Boltzmann was trying to explain why things always 'decay.' You always see a swinging pendulum damp, while its pivot point heats up a little from friction. You never see the pivot point cool down and the swinging increase. In coming up with a powerful theory to explain this all-important physical truth, Boltzmann was startled to encounter some examples where his equations predicted that there would not be damping—something you don't see in experiment!
"Boltzmann's intellectual opponents seized on this anomaly as evidence that his equations were wrong. But his equations are right. It's just that, back then, no one could do those particular experiments. These days, we can do the experiments. We all owe so much to Boltzmann and his legacy. We thought it would be a fitting tribute to him, to vindicate one of his more controversial (at the time!) predictions."
The reason why it has taken so long to experimentally confirm Boltzmann's prediction is because of the difficulty in generating a gas and confining it in space in a way that satisfies two strict requirements. One, the gas must be perfectly spherical (or "isotropic"), and two, it must confined by perfect harmonic confinement, which helps to reduce the effects of friction.
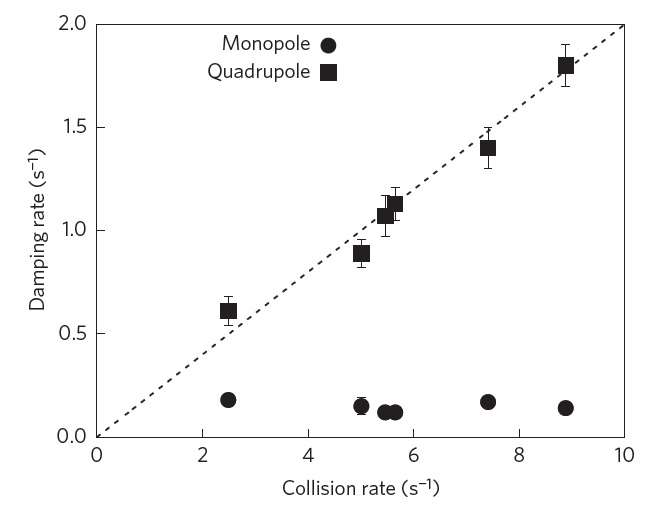
To realize such a system, the scientists used a new magnetic trap with extra magnetic coils, which allows various parameters to be adjusted independently. Using this device, the researchers trapped a cold gas cloud of rubidium atoms in such a way that the gas behaves in "monopole mode." In this mode, the temperature and cloud size of the gas oscillate with opposite phases—as one increases, the other decreases, and vice versa.
The scientists explain that these "breathing dynamics" are analogous to the oscillatory exchange between kinetic and potential energy that occurs in the simple harmonic motion of a swinging pendulum. Just as a swinging pendulum eventually reaches a state of equilibrium when it comes to a stop, a typical confined gas reaches a state of thermal equilibrium when heat ceases to flow. In both cases, the state of equilibrium is reached due to increasing entropy, which causes damping, or a decrease in the amplitude of the oscillations.
Here, however, the natural increase in entropy is frustrated by the very specific nature of the confinement and the interaction between the atoms. As evidence for this, the scientists showed that the gas in monopole mode barely experiences any damping at all, and the little bit of damping that it does experience is likely due to imperfections in the trap, since no physical system can provide perfectly isotropic and harmonic confinement. The researchers observed the greatly suppressed damping by taking images of the gas cloud from different angles and at very short intervals using phase-contrast microscopy, which allowed them to see oscillations in the cloud size.
Besides vindicating Boltzmann, the results could also have implications for understanding other non-equilibrium systems, including life itself.
"Non-equilibrium physics—the physics of what happens far away from thermal equilibrium—is a hot topic in science these days," Cornell said. "A classic example of matter out of equilibrium is life. How does it come about? Why does it persist? Our particular experimental example is maybe a little too clean, too classical, to be completely relevant to most modern research, but it is a great example of a broader physics idea called 'integrability' which explains why some systems don't ever achieve thermal equilibrium."
More information: D. S. Lobser, et al. "Observation of a persistent non-equilibrium state in cold atoms." Nature Physics. DOI: 10.1038/nphys3491
Journal information: Nature Physics
© 2015 Phys.org