Chromium's bonding angles let oxygen move quickly
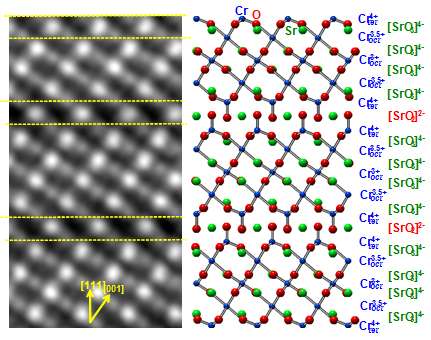
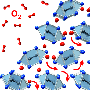
By taking advantage of the natural tendency of chromium atoms to avoid certain bonding environments, scientists at DOE's Pacific Northwest National Laboratory have generated a material that allows oxygen to move through it very efficiently, and at relatively low temperatures. Specifically, they found that their attempts to make metallic SrCrO3 lead instead to the formation of semiconducting SrCrO2.8. Because chromium as an ion with a charge of +4 does not like to form 90º bonds with oxygen, as it must in SrCrO3, SrCrO2.8 forms instead with a completely different crystal structure. This material contains oxygen-deficient planes through which oxygen can diffuse very easily.
"If oxygen vacancies are present at high enough concentrations, they can aggregate and form new ordered structures," said materials expert Dr. Scott Chambers, the PNNL Laboratory Fellow who led the research. "These ordered structures can have properties not observed in the host-creating novel, mesoscale crystals."
As a nation, we are always looking to create new and improved devices. Yet, the limits of what can be achieved with conventional materials, such as silicon-based electronics, are clearly on the horizon. This work represents an important scientific advancement relevant to increasing the efficiency of solid oxide fuel cells, which require oxides capable of absorbing and transmitting oxygen anions at low temperature.
"As an additional benefit, ordered arrays of oxygen vacancies might allow for the spatial separation of electronic and vibrational degrees of freedom," said Chambers. "This property would be useful in, for example, increasing the performance of thermoelectrics."
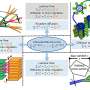
Using a combination of experimental and theoretical methods, scientists at PNNL made ultra-pure crystalline films and probed their properties. They used molecular beam epitaxy to prepare the films. To characterize the films, they used scanning transmission electron microscopy, electron energy loss spectroscopy, X-ray diffraction, X-ray and ultraviolet photoemission, optical absorption, and electrical transport. They used first-principles modeling to determine the structural transformations and oxygen anion diffusion kinetics.
They determined that the accumulation of oxygen vacancies in the cubic perovskite SrCrO3 (P-SCO) results in the formation of an ordered, nanostructured, rhombohedral phase, SrCrO2.8 (R-SCO). The rhombohedral version has quite different electronic and optical properties compared to P-SCO.
The team demonstrated that R-SCO can be reversibly oxidized to P-SCO under mild (500oC) and easily controlled experimental conditions, and that the R-SCO structure gives rise to much more facile oxygen ion conductivity than does P-SCO. This property is exceedingly important for solid oxide fuel cell technology, where oxygen reduction reaction kinetics and oxide ion conductivity in the cathode currently require high temperatures, around 800oC, which is a significant obstacle to improving the overall energy efficiency of fuel cells.
"This research can aid in the search for other similar structures with tailored characteristics," said Dr. Peter Sushko, a scientist who did the theoretical modeling and leads the Materials Science Group at PNNL.
In the near term, the team plans to apply the understanding gained to the deposition, characterization, and understanding of epitaxial strontium-doped lanthanum chromite. This material is of potential importance in visible light harvesting and, based on preliminary measurements at PNNL, may be a p-type transparent conducting oxide. In the long term, the team plans to exploit the observed phenomenon to carry out nanofabrication of novel heterogeneous catalytic structures by depositing submonolayer quantities of catalytically important metals on the surface of R-SCO, and using the intersection of the defect planes with the free surface to order the incoming metal atoms into nanowires.
More information: Zhang H, Y Du, PV Sushko, ME Bowden, RJ Colby, and SA Chambers. 2014. "Reversible Nano-Structuring of SrCrO3-δ Through Oxidization and Reduction at Low Temperatures." Nature Communications 5, Article number 4669. DOI: 10.1038/ncomms5669.
Journal information: Nature Communications
Provided by Oak Ridge National Laboratory