Study yields first snapshots of water splitting in photosynthesis
An international team, led by Arizona State University scientists, has published today in Nature a groundbreaking study that shows the first snapshots of photosynthesis in action as it splits water into protons, electrons and oxygen, the process that maintains Earth's oxygen atmosphere.
"This study is the first step towards our ultimate goal of unraveling the secrets of water splitting and obtaining molecular movies of biomolecules," said Petra Fromme, professor of chemistry and biochemistry at ASU. Fromme is the senior author and leader of the international team, which reported their work in "Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser," in the July 9 on-line issue of Nature.
Photosynthesis is one of the fundamental processes of life on Earth. The early Earth contained no oxygen and was converted to the oxygen-rich atmosphere we have today 2.5 billion years ago by the "invention" of the water splitting process in Photosystem II (PSII). All higher life on Earth depends on this process for its energy needs and PSII produces the oxygen we breathe, which ultimately keeps us alive.
The revealing of the mechanism of this water splitting process is essential for the development of artificial systems that mimic and surpass the efficiency of natural systems. The development of an "artificial leaf" is one of the major goals of the ASU Center for Bio-Inspired Solar Fuel Production, which was the main supporter of this study.
"A crucial problem facing our Center for Bio-Inspired Fuel Production (Bisfuel) at ASU and similar research groups around the world is discovering an efficient, inexpensive catalyst for oxidizing water to oxygen gas, hydrogen ions and electrons," said ASU Regents' Professor and Center Director Devens Gust. "Photosynthetic organisms already know how to do this, and we need to know the details of how photosynthesis carries out the process using abundant manganese and calcium.
"The research by Fromme and coworkers gives us, for the very first time, a look at how the catalyst changes its structure while it is working," Gust added. "Once the mechanism of photosynthetic water oxidation is understood, chemists can begin to design artificial photosynthetic catalysts that will allow them to produce useful fuels using sunlight."
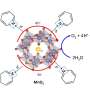
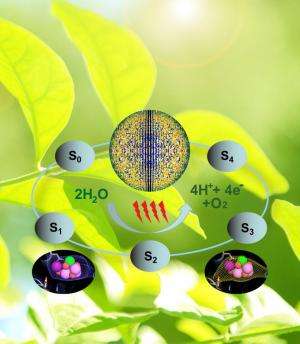
In photosynthesis, oxygen is produced at a special metal site containing four manganese atoms and one calcium atom connected together as a metal cluster. This oxygen-evolving cluster is bound to the protein PSII that catalyzes the light driven process of water splitting. It requires four light flashes to extract one molecule of oxygen from two water molecules bound to the metal cluster.
Fromme states that there are two major drawbacks to obtaining structural and dynamical information on this process by traditional X-ray crystallography. First, the pictures one can obtain with standard structural determination methods are static. Second, the quality of the structural information is adversely affected by X ray damage.
"The trick is to use the world's most powerful X-ray laser, named LCLS located at the Department of Energy's SLAC National Accelerator Laboratory," said Fromme. "Extremely fast femtosecond (10-15 second) laser pulses record snapshots of the PSII crystals before they explode in the X-ray beam, a principle called 'diffraction before destruction.'"
In this way, snapshots of the process of water splitting are obtained damage free. The ultimate goal of the work is to record molecular movies of water splitting.
The team performed the time-resolved femtosecond crystallography experiments on Photosystem II nanocrystals, which are so small that you can hardly see them even under a microscope. The crystals are hit with two green laser flashes before the structural changes are elucidated by the femtosecond X-ray pulses.
The researchers discovered large structural changes of the protein and the metal cluster that catalyzes the reaction. The cluster significantly elongates, thereby making room for a water molecule to move in.
"This is a major step toward the goal of making a movie of the molecular machine responsible for photosynthesis, the process by which plants make the oxygen we breathe, from sunlight and water," explained John Spence, ASU Regents' Professor of physics, team member and scientific leader of the National Science Foundation funded BioXFEL Science and Technology Center, which develops methods for biology with free electron lasers.
ASU recently made a large commitment to the groundbreaking work of the femtosecond crystallography team by planning to establish a new Center for Applied Structural Discovery at the Biodesign Institute at ASU. The center will be led by Petra Fromme.
Student role in research
An interdisciplinary team of eight ASU faculty members from the Department of Chemistry and Biochemistry (Petra Fromme, Alexandra Ros, Tom Moore and Anna Moore) and the Department of Physics (John Spence, Uwe Weierstall, Kevin Schmidt and Bruce Doak) worked together with national and international collaborators on this project. The results were made possible by the excellent work of current ASU graduate students Christopher Kupitz, Shibom Basu, Daniel James, Dingjie Wang, Chelsie Conrad, Shatabdi Roy Chowdhury, Jay-How Yang and ASU doctoral graduates and post-docs Kimberley Rendek, Mark Hunter, Jesse Bergkamp, Tzu-Chiao Chao and Richard Kirian.
Two undergraduate students Danielle Cobb and Brenda Reeder supported the team and gained extensive research experience by working hand in hand with graduate students, researchers and faculty at the free electron laser at Stanford. Four ASU senior scientists and postdoctoral researchers (Ingo Grotjohann, Nadia Zatsepin, Haiguang Liu and Raimund Fromme) supported the faculty in the design, planning and execution of the experiments, and were instrumental in evaluation of the data.
The first authorship of the paper is jointly held by the ASU graduate students Christopher Kupitz, who's dissertation is based on the development of new techniques for the growth and biophysical characterization of nanocrystals; and Shibom Basu, who devoted three years of his doctoral work to the development of the data evaluation methods.
"It is so exciting to be a part of this groundbreaking research and to have the opportunity to participate in this incredible international collaboration," said Kupitz, who will graduate this summer with a Ph.D. in biochemistry. "I joined the project because it fascinates me to work at the LCLS accelerator on this important biological project."
"The most exciting aspect of the work on Photosystem II is the prospect of making molecular movies to witness the water splitting process through time-resolved crystallography," added Basu.
National and international collaborators on the project include the team of Henry Chapman at DESY in Hamburg, Germany, who with the ASU team and researchers at the MPI in Heidelberg pioneered the new method of serial femtosecond crystallography. Other collaborators included a team led by Matthias Frank, an expert on laser spectroscopy and time-resolved studies with FELs at Lawrence Livermore National Laboratory, and the team of Yulia Pushkar at Purdue University, who supported the work with characterization of the crystals by electron paramagnetic resonance.
"We're tantalizingly close," said Chapman of the Center for Free-Electron Laser Science at DESY and a pioneer in X-ray free laser studies of crystallized proteins. "I think this shows that we really are on the right track and it will work."
More information: Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser, Nature, DOI: 10.1038/nature13453
Journal information: Nature
Provided by Arizona State University