December 10, 2013 report
Research team uses melanin to make biodegradable battery anode
(Phys.org) —A team of researchers from Carnegie Mellon University and the University of Oregon has used melanin as an ingredient in a cocktail that led to the creation of a biodegradable battery anode. In their paper published in Proceedings of the National Academy of Sciences, the team describes how they created the anode and to what purpose it might be put in the near future.
Over the past several decades, medical researchers have been working on a way to create devices that can remain inside the human body, without the risk of causing harm. One such example is the pace-maker. It sits inside the chest, monitoring heart activity and delivers a small jolt when needed to keep the heart beating on a proper rhythm. Though it's doubtless saved many lives, it also constitutes a health hazard—there are materials inside its case that upon escape could cause serious harm. Some of the most hazardous are in the battery, and that's why researchers are working so hard to find a way to build one that is not only non-toxic, but is also biodegradable. In this new effort, the researchers have taken a step towards that ultimate goal by creating a battery component that is made from a material that is created naturally by the human body—melanin.
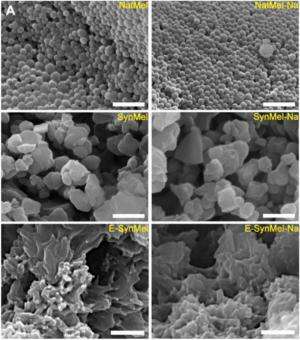
Melanin is a material that exists in the skin of humans and other animals, and is responsible for the creation of pigments. In its natural state it exists as a group of disordered extended hetero-aromatic polymer networks, which means it's a great material for holding a relatively large amount of ions. To use it as part of a battery, the team added it to a few other ingredients to create a mix that could be poured onto a steel mesh after adding sodium ions. The battery that resulted, the team found, was capable of discharging for up to five hours, though admittedly, offering less power than traditional batteries.
The entire battery was not biodegradable of course, which means much more work needs to be done before such a battery might be used with an implantable medical device. Possible uses for such a device range from monitoring wounds and/or blood pressure to the delivery of drugs to specific body parts.
More information: Biologically derived melanin electrodes in aqueous sodium-ion energy storage devices, PNAS, Published online before print December 9, 2013, DOI: 10.1073/pnas.1314345110
Abstract
Biodegradable electronics represents an attractive and emerging paradigm in medical devices by harnessing simultaneous advantages afforded by electronically active systems and obviating issues with chronic implants. Integrating practical energy sources that are compatible with the envisioned operation of transient devices is an unmet challenge for biodegradable electronics. Although high-performance energy storage systems offer a feasible solution, toxic materials and electrolytes present regulatory hurdles for use in temporary medical devices. Aqueous sodium-ion charge storage devices combined with biocompatible electrodes are ideal components to power next-generation biodegradable electronics. Here, we report the use of biologically derived organic electrodes composed of melanin pigments for use in energy storage devices. Melanins of natural (derived from Sepia officinalis) and synthetic origin are evaluated as anode materials in aqueous sodium-ion storage devices. Na+-loaded melanin anodes exhibit specific capacities of 30.4 ± 1.6 mAhg−1. Full cells composed of natural melanin anodes and λ-MnO2 cathodes exhibit an initial potential of 1.03 ± 0.06 V with a maximum specific capacity of 16.1 ± 0.8 mAhg−1. Natural melanin anodes exhibit higher specific capacities compared with synthetic melanins due to a combination of beneficial chemical, electrical, and physical properties exhibited by the former. Taken together, these results suggest that melanin pigments may serve as a naturally occurring biologically derived charge storage material to power certain types of medical devices.
Journal information: Proceedings of the National Academy of Sciences
© 2013 Phys.org