Clues to cocaine's toxicity could lead to better tests for its detection in biofluids
A new study on cocaine, the notorious white powder illegally snorted, injected or smoked by nearly 2 million Americans, details how it may permanently damage proteins in the body. That information, gleaned from laboratory tests, could be used to potentially detect the drug in biofluids for weeks or months—instead of days—after use, say scientists. The findings, which appear in the ACS journal Chemical Research in Toxicology, could also help explain cocaine's long-term health effects.
Anthony P. DeCaprio and colleagues explain that prescription and over-the-counter drugs intended for legal medical use undergo rigorous studies to determine how they work and how they might cause side effects. But there are very few similar studies on illicit drugs. Long-term use of the so-called rich man's drug is linked to depression, breathing problems, kidney diseases and sudden death. Researchers already knew that cocaine abuse can alter proteins in the body, but the exact details of how it makes these changes were not known. DeCaprio's team stepped in to investigate this mystery.
In laboratory tests, they discovered a brand-new way that cocaine breaks down and alters proteins. They speculate that these proteins could appear in users' biofluids for weeks or months after the drug is first taken. This finding could dramatically expand the window for determining past cocaine use, which currently is only detectable for up to several days. Also, the new details on cocaine metabolism contribute to a more comprehensive picture of the drug's toxic effects.
More information: "Covalent Thiol Adducts Arising from Reactive Intermediates of Cocaine Biotransformation" Chem. Res. Toxicol., Article ASAP. DOI: 10.1021/tx4003116
Abstract
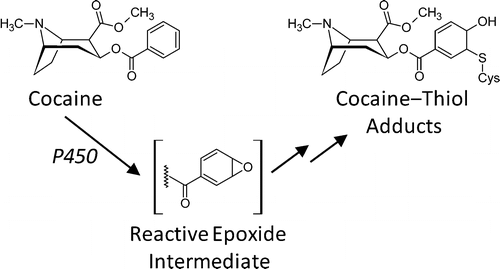
Exposure to cocaine results in the depletion of hepatocellular glutathione and macromolecular protein binding in humans. Such cocaine-induced responses have generally been attributed to oxidative stress and reactive metabolites resulting from oxidative activation of the cocaine tropane nitrogen. However, little conclusive data exists on the mechanistic pathways leading to protein modification or the structure and specificity of cocaine-derived adduction products. We now report a previously uncharacterized route of cocaine bioactivation leading to the covalent adduction of biological thiols, including cysteine and glutathione. Incubation of cocaine with biological nucleophiles in an in vitro biotransformation system containing human liver microsomes identified a monooxygenase-mediated event leading to the oxidation of, and subsequent sulfhydryl addition to, the cocaine aryl moiety. Adduct structures were confirmed using ultra-high performance liquid chromatography coupled to high resolution, high mass accuracy mass spectrometry. Examination of assays containing transgenic bactosomes expressing single human cytochrome P450 isoforms determined the role of P450s 1A2, 2C19, and 2D6 in the oxidation process resulting in adduct formation. P450-catalyzed aryl epoxide formation and subsequent attack by free nucleophilic moieties is consistent with the resulting adduct structures, mechanisms of formation, and the empirical observation of multiple structural and stereo isomers. Analogous adduction mechanisms were maintained across all sulfhydryl-containing nucleophile models examined; N-acetylcysteine, glutathione, and a synthetic cysteine-containing hexapeptide. Predictive in silico calculations of molecular reactivity and electrophilicity/nucleophilicity were compared to the results of in vitro assay incubations in order to better understand the adduction process using the principles of hard and soft acid and base (HSAB) theory. This study elucidated a novel metabolic pathway that may be of particular significance to the clinical and forensic toxicology of cocaine and provides analytical tools and methods that can be applied to the determination of these conjugates in humans, opening a new area of research on cocaine biotransformation and toxicology.
Journal information: Chemical Research in Toxicology
Provided by American Chemical Society















