New material holds big energy hope

(Phys.org) —A new material that can store large amounts of energy with very little energy loss has been developed by researchers at the Australian National University.
The material has practical applications in renewable energy storage, electric cars and defence and space technologies.
"Dielectric materials are used to make fundamental electrical components called capacitors, which store energy," said Associate Professor Yun Liu of the ANU Research School of Chemistry, co-author of the paper detailing the new material.
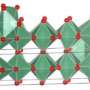
The new metal oxide dielectric material outperforms current capacitors in many aspects, storing large amounts of energy and working reliably from -190°C to 180°C, and is cheaper to manufacture than current components.
"Our material performs significantly better than existing high dielectric constant materials so it has huge potential. With further development, the material could be used in 'supercapacitors' which store enormous amounts of energy, removing current energy storage limitations and throwing the door wide open for innovation in the areas of renewable energy, electric cars, even space and defence technologies," said Associate Professor Liu.
The material could be particularly transformative for wind and solar power, which can cause problems when fed into the power grid at low demand times.
"Power going into the grid has to balance with the demand for power at any given time," said co-author Professor Ray Withers. "This means that it is very important to be able to store energy until such time as it is really needed."
Researchers have been trying to design new dielectric materials to make more efficient energy storage devices for years.
The design process has proven difficult because the materials need to meet three requirements: a very high dielectric constant, meaning they can store a lot of energy; a very low dielectric loss, meaning energy doesn't leak out and get wasted; and the capacity to work across a broad range of temperatures.
"If you have a higher dielectric constant but also a high loss, the material is basically useless because it doesn't store energy well – it's like a leaky bucket. The material would also be useless if it only performs well at a certain temperature, because it couldn't deal with normal daily temperature fluctuations. It is very difficult to achieve all three of these features," said Professor Withers.
After five years of hard work, the research team has developed a material that meets all these requirements.
"Our success was a mixture of luck, experimentation and determination," said Associate Professor Liu. "When we first found this material we knew it had great potential. It's friendly to the environment, non-toxic and abundant."
More information: Electron-pinned defect-dipoles for high-performance colossal permittivity materials, Nature Materials (2013) doi:10.1038/nmat3691 , www.nature.com/nmat/journal/va … t/full/nmat3691.html
Journal information: Nature Materials
Provided by Australian National University