New study reveals chemical transformations of ambient organic aerosols
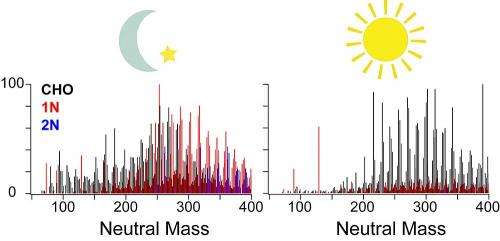
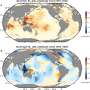
Deciphering the molecular composition of organic aerosols, or OA, in the atmosphere is essential for understanding how these complex aerosols transform and impact the environment and climate forcing. The chemical composition of OA influences how atmospheric radiation is absorbed. For example, the presence of nitrogen-containing organic compounds (NOC) in OA may have a profound effect on its light-absorption properties, affecting how OA influences regional haze and Earth's climate. OA sources, their atmospheric transformation, and radiative effects are core sources of uncertainty in today's atmospheric climate models. At present, the understanding of OA composition is limited to the extent that their impact cannot be accurately predicted and mitigated.
To probe the chemical compositional changes that occur in OA during a diurnal cycle and study the distribution of unique OA species across multiple scales, scientists employed EMSL's nanospray desorption electrospray ionization (nano-DESI) coupled with high-resolution mass spectrometry (HR-MS) capabilities to characterize more than 850 unique molecular species in the study area. Nano-DESI/HR-MS enables fast, sensitive direct analysis of minute amounts of the OA samples deposited on substrates and affords an accurate assessment of the transforming reactions and their plausible causes. The analyses revealed patterns, such as increased NOC in samples taken at night with more OA containing only carbon, hydrogen, and oxygen (CHO) in the afternoon. High ozone concentrations correlated with the predominant CHO compounds, indicating they were secondary products of daytime oxidation, including ozonolysis and/or photochemical reactions.
Meanwhile, the observed nighttime enhancement of NOC showed evidence of being formed by reactions that transform carbonyls into imines, which require aqueous-phase chemistry of ammonium and organic compounds in liquid-phase particles. Thus, the scientists hypothesized ammonium chemistry may be an important pathway for NOC formation in OA. In addition to providing an alternate technique for investigating OA, and potentially more precise models, this work is another step toward clarifying environmental and climate change ramifications that stem from the chemical transformations that determine OA composition.
More information: O'Brien, R. et al. 2013. Molecular characterization of organic aerosol using nanospray desorption/electrospray ionization mass spectrometry: CalNex 2010 field study, Atmospheric Environment 68:265-272. DOI: 10.1016/j.atmosenv.2012.11.056.
Journal information: Atmospheric Environment
Provided by Environmental Molecular Sciences Laboratory