Random walks on DNA
Scientists have revealed how a bacterial enzyme has evolved an energy-efficient method to move long distances along DNA. The findings, published in Science, present further insight into the coupling of chemical and mechanical energy by a class of enzymes called helicases, a widely-distributed group of proteins, which in human cells are implicated in some cancers.
The new helicase mechanism discovered in this study, led by researchers from the University of Bristol and the Technische Universität Dresden in Germany, may help resolve some of the unexplained roles for helicases in human biology, and in turn help researchers to develop future technological or medical applications.
A commonly held view of DNA helicases is that they move along DNA and "unzip" the double helix to produce single strands of DNA for repair or copying. This process requires mechanical work, so enzyme movement must be coupled to consumption of the chemical fuel ATP. These enzymes are thus often considered as molecular motors.
In the new work, Ralf Seidel and his team at the Technische Universität Dresden developed a microscope that can stretch single DNA molecules whilst at the same time observe the movement of single fluorescently-labelled helicases. In parallel, the Bristol researchers in the DNA-Protein Interactions Unit used millisecond-resolution fluorescence spectroscopy to reveal dynamic changes in protein conformation and the kinetics of ATP consumption.
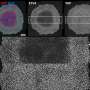
The team studied a helicase found in bacteria that moves along viral (bacteriophage) DNA. The work demonstrated that, surprisingly, the enzyme only consumed ATP at the start of the reaction in order to change conformation. Thereafter long-range movement along the DNA was driven by thermal motion; in other words by collisions with the surrounding water molecules. This produces a characteristic one-dimensional "random walk" (see picture), where the protein is just as likely to move backwards as forwards.
Mark Szczelkun, Professor of Biochemistry from the University's School of Biochemistry and one of the senior authors of the study, said: "This enzyme uses the energy from ATP to force a change in protein conformation rather than to unwind DNA. The movement on DNA thereafter doesn't require an energy input from ATP. Although movement is random, it occurs very rapidly and the enzyme can cover long distances on DNA faster than many ATP-driven motors. This can be thought of as a more energy-efficient way to move along DNA and we suggest that this mechanism may be used in other genetic processes, such as DNA repair."
More information: "The helicase-like domains of Type III restriction enzymes trigger long-range diffusion along DNA" by Friedrich W. Schwarz et al. Science, 2013. www.sciencemag.org/content/340/6130/353.abstract
Journal information: Science
Provided by University of Bristol