January 9, 2013 report
Researchers create super-repellant surface material (w/ video)
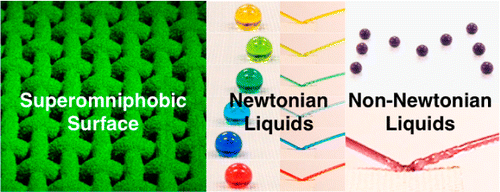
(Phys.org)—Researchers from the University of Michigan working in collaboration with associates from the US Air Force have created a new type of surface cover that repels oils, water, alkali solutions, acids and even non-Newtonian fluids. In their paper published in Journal of the American Chemical Society, the researchers describe their new material and the different ways it can repel various liquids.
The material they've created works due to two separate aspects: its chemical structure and its physical layout. It's based on a very small gauge steel mesh which has been coated with polymer (PDMS and POSS) beads. The unique pattern laid down limits surface area and has an overhanging structure that limits adhesion. Also, tiny air pockets between the beads prevent materials from actually touching other parts of the surface, preventing liquids from getting a grip.
The researchers explain that surface repellents work in general by limiting the wetting hysteresis – the amount of deformation that occurs when a liquid hits a surface. Ideally the contact angle at both the front and rear of a drop remain the same – the result is a lessened impact area. In practical terms this means that the more a drop remains formed like a drop when it strikes, the less likely it is to adhere to a surface.
Researchers have found it particularly difficult to develop surface covers that repel liquids that contain polymers, particularly non-Newtonian fluids. This is because such substances tend to deform almost immediately on contact. Thus, the challenge has been to discover a way to cause such fluids to retain their shape as they drop onto a surface. With the new material, the overhanging, eave-like edges of the beads prevent the liquid drop from distending while also preventing it from reaching an adjacent part of the surface. That limits the amount of distension and thus the deformation of the drop.
The researchers demonstrated the material's ability to repel liquids by shooting various liquids through a small jet at a covered surface and filming it as it bounced off instead of adhered. They also demonstrated that the covering also provides protection from chemical attack by dunking a coated aluminum plate into several acidic solutions. Its strength in doing so, the team explains, comes about from the same properties that prevent adhesion. If an acid cannot touch a surface (because of the air pockets) it cannot destroy it.
More information: Superomniphobic Surfaces for Effective Chemical Shielding, J. Am. Chem. Soc., Article ASAP, DOI: 10.1021/ja310517s
Abstract
Superomniphobic surfaces display contact angles >150° and low contact angle hysteresis with essentially all contacting liquids. In this work, we report surfaces that display superomniphobicity with a range of different non-Newtonian liquids, in addition to superomniphobicity with a wide range of Newtonian liquids. Our surfaces possess hierarchical scales of re-entrant texture that significantly reduce the solid–liquid contact area. Virtually all liquids including concentrated organic and inorganic acids, bases, and solvents, as well as viscoelastic polymer solutions, can easily roll off and bounce on our surfaces. Consequently, they serve as effective chemical shields against virtually all liquids—organic or inorganic, polar or nonpolar, Newtonian or non-Newtonian.
Journal information: Journal of the American Chemical Society
© 2013 Phys.org