PEFT: Clever thinking creates cleaner water

Two chemists from the University of Waikato have come up with an innovative method for treating bore water on Waikato farms.
They're currently trialling the system on a Waikato farm and may have hit upon a low-cost solution for developing countries, where many people have limited access to clean and affordable water.
Using electrochemistry
Associate Professor Alan Langdon and post-doctoral researcher Dr Hilary Nath decided to try using electrochemistry to remove the iron and manganese prevalent in bore water from Waikato's peaty soils.
The residues give the water its typical browny-orange colour, and generally make it undrinkable without expensive treatment using aerators, filters, ion exchangers and tanks.
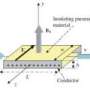
The researchers came up with a simple system that uses electric current passing between two perforated titanium electrodes to turn naturally occurring chloride ions in the water into chlorine.
The chlorine then oxidises and precipitates out the metal contaminants, and also disinfects the water passing through the system, making it safe to drink.
Best of all, the whole system can be powered by a car battery.
"By bringing the electrodes closer together than anyone else has been able to we can reduce electrical resistance and consume less power," says Dr Nath. "And because the flow path through the cell is very short, we can achieve good water flow at modest pressure."
PEFT System
The system is known as PEFT – perforated electric flow through – and is patented in New Zealand with international patents filed. A prototype will be on show at the University of Waikato stand at Equidays next month; the university is a strategic partner of Equidays which runs from 2-4 November.
Drs Langdon and Nath are now testing the prototype, and getting good results – they've seen total oxidation of iron during their trial.
"The initial focus will be disinfection of harvested rain water, disinfection of water supplies derived from surface water and bore water contaminated with iron – we need to be very sure our technology is robust before contemplating overseas markets, particularly in developing nations."
The researchers noticed that the closer together the two electrodes were positioned, the higher the electric field generated between them. And the higher the electric field, the more potent the chlorine being produced.
Clever technology
The two together were so powerful they could kill bugs in the water at much lower chlorine levels than normally required – the electric field was able to puncture the membrane of a bug making it 100 times more susceptible to the disinfecting effect of the chlorine.
At slightly higher applied voltages the PEFT cell can also disinfect water by the electric field alone, with no need to produce any chlorine.
"It's low technology, but it's very clever nevertheless," says Dr Langdon.
Provided by University of Waikato