Freezing water droplets form sharp ice peaks
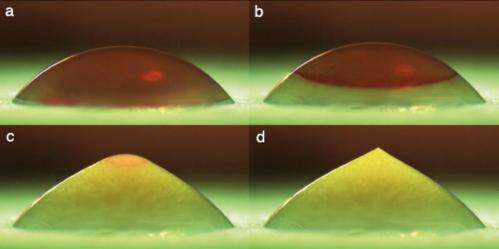
Photos reveal how water droplets placed on a cold surface freeze to a sharp point that sprouts a "forest" of tree-like ice crystals.
Researchers at the University of Twente, in the Netherlands, placed water droplets on a plate chilled to -20 degrees Celsius and captured images as a freezing front traveled up the droplet.
The photos are published in the American Institute of Physics' (AIP) journal Physics of Fluids. The approximately 4-millimeter diameter droplets took about 20 seconds to freeze. During the final stage of freezing, the ice drop developed a pointy tip, as can be seen in Figure 1d. The effect, which is not observed for most other liquids, arises because water expands as it freezes. The vertical expansion of the ice, in combination with the confining effect of surface tension on the spherical cap of remaining liquid, leads to the point formation.
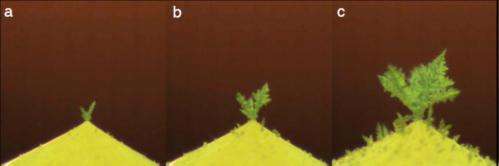
Once the liquid is completely frozen, the sharp tip of the drop attracts water vapor in the air, much like a sharp metal lightning rod attracts electrical charges. The water vapor collects on the tip and a tree of small ice crystals starts to grow, as seen in Figure 2. An opposite effect has been shown to preferentially extract water molecules from the sharp edge of potato wedges in the oven, the researchers note.
More information: "Freezing singularities in water drops" is published in Physics of Fluids. pof.aip.org/resource/1/phfle6/v24/i9/p091102_s1
Journal information: Physics of Fluids
Provided by American Institute of Physics



















