Paints and coatings containing bactericidal agent nanoparticles combat marine fouling
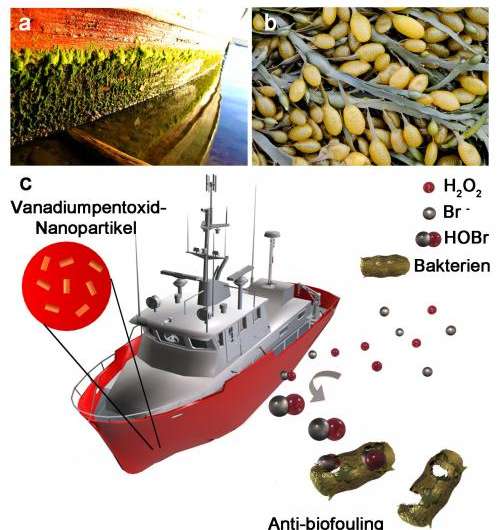
Scientists at Johannes Gutenberg University Mainz (JGU) in Germany have discovered that tiny vanadium pentoxide nanoparticles can inhibit the growth of barnacles, bacteria, and algae on surfaces in contact with water, such as ship hulls, sea buoys, or offshore platforms. Their experiments showed that steel plates to which a coating containing dispersed vanadium pentoxide particles had been applied could be exposed to seawater for weeks without the formation of deposits of barnacles, bacteria, and algae. In comparison, plates that were coated only with the ship's normal paint exhibited massive fouling after exposure to seawater for the same period of time. The discovery could lead to the development of new protective, antifouling coatings and paints that are less damaging to the environment than the ship coatings currently used.
Marine fouling is a problem that costs the shipping industry more than 200 billion dollars per year. The accumulation of organisms such as algae, mussels, and barnacles increases the objects' water resistance and, in consequence, fuel consumption. This means additional costs for shipping companies and, even worse, increased environmental damage due to extra CO2 emissions. Within only a few months, an underwater boat hull can be completely covered and overgrown with organisms. According to Lloyds, this means an increase in fuel consumption of up to 28 percent and about 250 million tons of additional CO2 emissions per year. While it is possible to counteract this effect to some extent by means of the use of antifouling paints, conventional biocides are less effective and can have adverse environmental consequences. In addition, microorganisms can develop resistance to them.

It was one of nature's own defense mechanisms that provided the inspiration for the approach now taken by the team of scientists working under Professor Dr. Wolfgang Tremel of the Institute of Inorganic Chemistry and Analytical Chemistry at JGU. Certain enzymes found in brown and red algae produce halogen compounds that have a biocidal potential. It is assumed that these are synthesized by the algae to protect them against microbial attack and predators. The chemists at Mainz University decided to mimic this process using vanadium pentoxide nanoparticles. According to their article published in Nature Nanotechnology, vanadium pentoxide (V2O5) nanoparticles have "an intrinsic biomimetic bromination activity […] which makes them a practical and cost-efficient alternative for conventional chemical biocides." Vanadium pentoxide functions as a catalyst so that hydrogen peroxide and bromide combine to form small quantities of hypobromous acid, which is highly toxic to many microorganisms and has a pronounced antibacterial effect. The required reactants are present in seawater: This already contains bromide ions, while small quantities of hydrogen peroxide are formed when it is exposed to sunlight.
The process has been demonstrated both under laboratory conditions and in natural seawater. It has only very minimal consequences for the environment because the effect is restricted to micro-surfaces. The metallic oxide is particularly potent when it is present in the form of nanoparticles because then, due to the larger surface area, there is an enhanced catalytic effect.
"Vanadium pentoxide nanoparticles, due to their poor solubility and the fact that they are embedded in the coating, are considerably less toxic to marine life than are the tin- and copper-based active substances used in the commercially available products," explains Wolfgang Tremel. In his view, ships' coatings based on vanadium pentoxide could be a practical and cost-effective alternative to conventional chemical biocides. "Here we have an environmentally-compatible component for a new generation of antifouling paints that employ the natural defense mechanism used by marine organisms."
Ron Wever, the team's Dutch cooperation partner from the University of Amsterdam, has been investigating such natural defense mechanisms for the last 15 years. He suggested adding the enzyme involved, i.e., vanadium haloperoxidase, to antifouling paints. The chemists in Mainz are now working together with Wever to develop vanadium pentoxide nanoparticles. "Vanadium pentoxide particles are considerably cheaper and also more stable than genetically produced enzymes," he adds.
A research group headed by Dr. Klaus Peter Jochum of the Max Planck Institute for Chemistry in Mainz has been conducting experiments to determine whether the use of vanadium pentoxide might have a negative effect on the environment. Using a highly sensitive ICP mass spectrometer, the scientists determined the concentration of vanadium in various samples of seawater that had been exposed to the coated material for different lengths of time. The results showed that levels were only slightly elevated above the normal average vanadium concentration in seawater. It can thus be concluded that only very tiny amounts of vanadium migrate from the coating into seawater and will thus have no negative impact on the environment.
More information: F. Natalio et al., Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation, Nature Nanotechnology, Advance Online Publication, 1 July 2012, doi:10.1038/NNANO.2012.91
Journal information: Nature Nanotechnology
Provided by Universitaet Mainz