A cleaner, faster battery
A team of Stanford and Canadian Light Source researchers have developed an ultrafast rechargeable battery from non-toxic materials.
This new rechargeable battery uses nanostructures to update a century-old idea: the nickel-iron bat-tery. Those early batteries, developed by Thomas Edison in 1901 to power electric cars, have been out of favour for a while. In addition to taking a long time to recharge, they just weren’t as powerful as other sources.
There are, however, compelling reasons to improve the batteries’ efficiency.
“Nickel-iron batteries are attractive because they are cheap and, relative to other battery materials like lithium, they are not very toxic,” explained Dr. Jigang Zhou, a Canadian Light Source Industry Scientist who worked on the project.
Stanford’s Dr. Hongjie Dai and his research team worked with CLS scientists as well as a researcher in Beijing to develop a much faster nickel-iron battery.
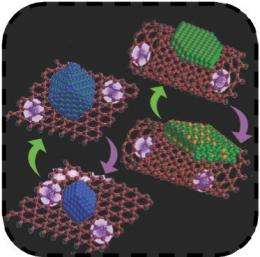
The new battery charges in less than two minutes, thanks to a newly developed carbon nanostructure. To achieve the desired conductivity, the team grew nanocrystals of iron oxide on thin sheets of car-bon, and nickel nanocrystals on carbon nanotubes. Traditional batteries lack this structure, mixing iron and nickel with conductors more or less randomly.
The result was a strong chemical bond between the materials, which the team identified and studied at the Canadian synchrotron. The CLS team also included Staff Scientist Tom Regier and Research Associate Jian Wang, who lent their synchrotron expertise to the Stanford team.
“The title of the paper has the term ‘strongly coupled,’ and this is important. This strongly coupled material is needed to deliver ultrafast [batteries],” Zhou said.
Strong coupling between materials makes it much easier to pass electrons -- the currency of electric flow -- just as it’s faster to give someone money when they’re sitting right next to you, than to have to cross the room to do it. The smaller the gap, or the more strongly coupled the materials are, the faster electrons move.
“You can only check if it is strongly coupled by the technique that we did here. There are other beam-lines that can do, in principle, similar work, but this is one of the best soft X-ray beamlines in the world.”
So as a result of the strong coupling identified and studied at the synchrotron, these batteries are able to charge and discharge ultrafast. However, the batteries have a limited life. They can only be re-charged about 800 times before their usable life is over.
Zhou hopes to study other potential benefits to these strongly coupled materials, and gather more information about their structures and how they change over time. Future work to understand structur-al changes over time will help researchers develop a longer-lasting, and potentially even faster, ver-sion of the battery.
This work represents a small part of the possible uses of synchrotron science in developing new energy applications and exploring new materials.
Provided by Canadian Light Source