Study describes molecular machinery that pulls apart protein clumps
Amyloid fibers are protein aggregates associated with numerous neurodegenerative diseases, including Parkinson's disease, for which there are no effective treatments.
However, on the flip side, the fibers can also play beneficial, protective roles. In yeast, they are associated with increased survival and the evolution of new traits. In humans, they form biological nanostructures to house pigments and other molecules and may also be central to long-term memory formation and storage. Amyloid fibers are among the most stable protein-based structures in nature, and so when they are detrimental, as in Parkinson's disease, they are notoriously difficult for cells to break down.
In a new study published in PLoS Biology this week, James Shorter, PhD, assistant professor of Biochemistry and Biophysics, Perelman School of Medicine at the University of Pennsylvania, and colleagues address an urgent need to find ways to promote beneficial amyloid fiber assembly or to reverse its pathogenic assembly, at will.
In the paper, Shorter and colleagues define the mechanisms by which small heat-shock proteins (hsp) collaborate with other molecular chaperones to regulate the assembly and disassembly of a beneficial yeast prion (an amyloid that can spread between individuals).
Yeast harbor a declumping protein called Hsp104, which rapidly disassembles amyloid fibers, and this activity is greatly enhanced by small heat shock proteins. Humans and other animals, however, lack Hsp104, and so the puzzle has always been: Can human cells also disassemble these exceptionally stable amyloid fibers?
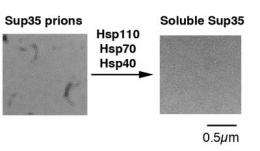
In this study, Shorter and colleagues establish that in the absence of Hsp104, the yeast small heat shock proteins collaborate with other molecular chaperones to slowly depolymerize the amyloid fibers by removing one fiber subunit at a time from the tips of the fibers. This activity was extremely surprising as traditionally the small heat shock proteins and other molecular chaperones are famous for their duties in preventing protein clumping. They were not previously known to reverse the assembly of exceptionally stable amyloid fibers that were already formed.
Importantly, the proteins of the amyloid-depolymerase machinery is conserved to humans. Thus, even without Hsp104, human small heat shock proteins can collaborate with human molecular chaperones to slowly depolymerize amyloid fibers. For example, it is now clear that human cells harbor the necessary machinery to clear amyloid fibers connected with neurodegenerative disease.
"Remarkably, the human small heat shock protein, HspB5, stimulates other heat shock proteins, Hsp110, Hsp70, and Hsp40, to gradually depolymerize amyloid fibers formed by alpha-synuclein, which are implicated in Parkinson's disease, from their ends on a biologically relevant timescale. Because monomers [shorter segments] are released by this system and not toxic oligomers [longer segments], we believe this is an extremely safe way to dissolve amyloid" explains Shorter.
This newly identified and highly conserved amyloid-depolymerase system could have important therapeutic applications for various neurodegenerative disorders, suggest the researchers. The goal is to stimulate the machinery in humans to pull apart the fibers where and when needed by increasing the expression of heat shock proteins to hopefully pull apart already-formed amyloid fibers. The next step will be to boost the activity of the newly discovered amyloid-depolymerase system, perhaps with drug-like small molecules, in animal models of neurodegenerative disease.
More information: Duennwald ML, Echeverria A, Shorter J (2012) Small Heat Shock Proteins Potentiate Amyloid Dissolution by Protein Disaggregases from Yeast and Humans. PLoS Biol 10(6): e1001346. doi:10.1371/journal.pbio.1001346
Journal information: PLoS Biology
Provided by University of Pennsylvania School of Medicine