June 15, 2012 report
German team finds a way to link boron atoms with a triple bond
(Phys.org) -- In the chemical world, there are few instances where atoms form triple bonds (where three electrons from an atom are bonded with the electrons from another atom). In fact other than triple bonds between carbon and nitrogen few examples exist at all. One instance that theory had predicted should work was with boron, mainly because of where it sits on the Periodic Table of Elements, but also of course because in its stable state it has just three electrons. Because of this, various chemists over the years have tried to figure out a way to form a compound made of triple bonded boron atoms. Now, it appears a team in Germany at the University of Würzberg, has succeeded. They have published a paper describing their results in the journal Science.
Two get two boron atoms to triple bond with one another, you can’t just shove them together and hope they will stick because they are too stable in their current state. In other words, they’ll just ignore one another. To get them to bond some external force must be introduced. Prior researchers had tried using a laser as a force, but only succeeded in creating a triple bound that could exist at eight degrees above absolute zero. At room temperature it vaporized.
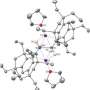
The German team took another approach, instead of trying to push the atoms into bonding, they coaxed them together by slowly filling the places that the three electrons can exist around the nucleus with something else, essentially leaving them no choice but to bond with the electrons from another boron atom. To do that, they introduced a molecule that contained carbon and nitrogen atoms, called a N-heterocyclic carbine (NHC) in a sterile vacuum chamber. The idea is that because main-group elements such as boron are most stable when surrounded by eight electrons, creating an environment where the boron atoms could share two electrons from the NHC would leave three openings for electrons from one boron atom to be shared with those of another. Thus, the three electrons from each would form bonds with each other naturally to create the full complement of eight electrons. To make it happen they induced a boron-boron double bond as a first step, and then a triple bond in the second.
The result is a green crystal that will exist at room temperature indefinitely in the absence of air or water. Because the discovered process and resultant material is still so new, scientists don’t really know if it might be useful for anything, but the fact that the team has finally solved the riddle of how to get boron to triple bound, will assure them a place in the history books.
More information: Ambient-Temperature Isolation of a Compound with a Boron-Boron Triple Bond, Science, 15 June 2012: Vol. 336 no. 6087 pp. 1420-1422. DOI: 10.1126/science.1221138
ABSTRACT
Homoatomic triple bonds between main-group elements have been restricted to alkynes, dinitrogen, and a handful of reactive compounds featuring trans-bent heavier elements of groups 13 and 14. Previous attempts to prepare a compound with a boron-boron triple bond that is stable at ambient temperature have been unsuccessful, despite numerous computational studies predicting their viability. We found that reduction of a bis(N-heterocyclic carbene)-stabilized tetrabromodiborane with either two or four equivalents of sodium naphthalenide, a one-electron reducing agent, yields isolable diborene and diboryne compounds. Crystallographic and spectroscopic characterization confirm that the latter is a halide-free linear system containing a boron-boron triple bond.
Journal information: Science
© 2012 Phys.Org