Sulfur in every pore: Improved batteries with carbon nanoparticles
From smartphones to e-bikes, the number of mobile electronic devices is steadily growing around the world. As a result, there is an increased need for batteries that are small and light, yet powerful. As the potential for the further improvement of lithium-ion batteries is nearly exhausted, experts are now turning to a new and promising power storage device: lithium-sulfur batteries.
In an important step toward the further development of this type of battery, a team led by Professor Thomas Bein of LMU Munich and Linda Nazar of Waterloo University in Canada has developed porous carbon nanoparticles that utilize sulfur molecules to achieve the greatest possible efficiency.
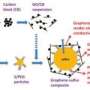
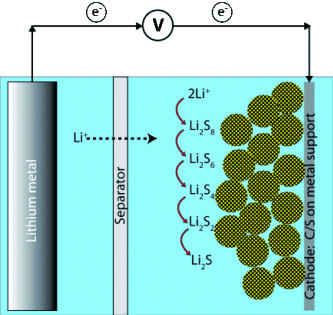
In prototypes of the lithium-sulfur battery, lithium ions are exchanged between lithium- and sulfur-carbon electrodes. The sulfur plays a special role in this system: Under optimal circumstances, it can absorb two lithium ions per sulfur atom. It is therefore an excellent energy storage material due to its low weight. At the same time, sulfur is a poor conductor, meaning that electrons can only be transported with great difficulty during charging and discharging. To improve this battery's design the scientists at Nanosystems Initiative Munich (NIM) strive to generate sulfur phases with the greatest possible interface area for electron transfer by coupling them with a nanostructured conductive material.
To this end, Thomas Bein and his team at NIM first developed a network of porous carbon nanoparticles. The nanoparticles have 3- to 6-nanometer wide pores, allowing the sulfur to be evenly distributed. In this way, almost all of the sulfur atoms are available to accept lithium ions. At the same time they are also located close to the conductive carbon.
"The sulfur is very accessible electrically in these novel and highly porous carbon nanoparticles and is stabilized so that we can achieve a high initial capacity of 1200 mAh/g and good cycle stability," explains Thomas Bein. "Our results underscore the significance of nano-morphology for the performance of new energy storage concepts."
The carbon structure also reduces the so-called polysulfide problem. Polysulfides form as intermediate products of the electrochemical processes and can have a negative impact on the charging and discharging of the battery. The carbon network binds the polysulfides, however, until their conversion to the desired dilithium sulfide is achieved. The scientists were also able to coat the carbon material with a thin layer of silicon oxide which protects against polysulfides without reducing conductivity.
Incidentally, the scientists have also set a record with their new material: According to the latest data, their material has the largest internal pore volume (2.32 cm3/g) of all mesoporous carbon nanoparticles, and an extremely large surface area of 2445 m2/g. This corresponds roughly to an object with the volume of a sugar cube and the surface of ten tennis courts. Large surface areas like this might soon be hidden inside our batteries.
More information: "Spherical Ordered Mesoporous Carbon Nanoparticles with Extremely High Porosity for Lithium-Sulfur Batteries". Jörg Schuster, Guang He, Benjamin Mandlmeier, Taeeun Yim, Kyu Tae Lee, Thomas Bein and Linda F. Nazar. Angewandte Chemie, 1 MAR 2012. onlinelibrary.wiley.com/doi/10 … e.201107817/abstract
Journal information: Angewandte Chemie
Provided by Ludwig-Maximilians-Universitat Munchen