December 7, 2011 feature
Superhard carbon material could crack diamond
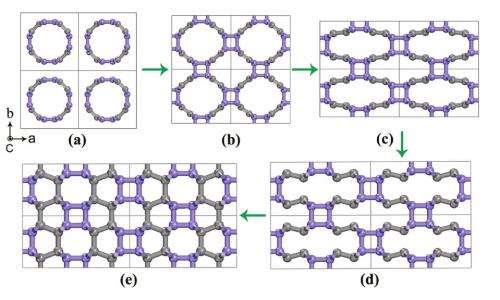
(PhysOrg.com) -- By applying extreme pressure to compress and flatten carbon nanotubes, scientists have discovered that they can create a new carbon polymer that simulations show is hard enough to crack diamond. The pressure-induced formation process of the new carbon allotrope, called Cco-C8, is similar to the 3D polymerization of the soccer-ball-like buckminsterfullerene, C60, at high pressure. When the carbon nanotube bundle is subjected to further compression, it becomes even more distorted and flattened to produce the Cco-C8 structure.
The scientists, led by Professor Yongjun Tian from the State Key Laboratory of Metastable Materials Science and Technology at Yanshan University in Qinhuangdao, China, have published their study on the new superhard carbon in a recent issue of Physical Review Letters.1
“Star material carbon exists in various architectures due to its ability to form sp-, sp2-, and sp3-hybridized bonds, fostering graphite, diamond, lonsdaleite, carbine, chaoite, amorphous carbon, nanotubes, fullerenes, graphene, and so on,” Tian told PhysOrg.com. “These carbon allotropes possess outstanding and unparalleled properties, as well as unique scientific and technological importance, such that searching for new carbon allotropes has long been a hot topic in scientific research communities. The greatest significance of this work lies in the new strategy of directly compressing carbon nanotube bundles to design and to synthesize novel metastable carbon allotropes. This strategy implies that some metastable phases of carbon with higher energy may also be experimentally obtained.”
As the scientists explain, applying pressure to some of these carbon allotropes can change the bonds, resulting in different forms of carbon with novel electronic and mechanical properties.
Instead of experimentally searching for new carbon allotropes, the scientists here used a recently developed technique called the Crystal Structure Analysis by Particle Swarm Optimization (CALYPSO). This computerized search was designed to predict stable crystal structures using only chemical compositions of a given compound at specified external conditions, such as pressure.
The CALYPSO simulations first yielded several carbon structures that are already experimentally known (such as graphite and diamond) or theoretically proposed (such as chiral C6). The simulations then revealed the novel Cco-C8 allotrope, a 3D polymer composed of thin (2, 2) carbon nanotubes interconnected through 4-and 6-member carbon rings, which arises due to the formation of additional bonds between carbon atoms.
The simulations showed that Cco-C8 has a Vickers hardness of 95.1 GPa, which is slightly below diamond’s 97.5 GPa. Although there are several ways to measure the hardness of a material, Vickers hardness is one of the most common methods. In this method, a sharp object is compressed into a material, and the dimensions of the resulting indentation are measured.
“Hardness has been used as one of the macroscopic mechanical properties of materials for about three centuries,” Tian explained. “Usually, hardness can be defined macroscopically as the ability of a material to resist being scratched or dented by another. Recently, we defined hardness microscopically as the combined resistance of chemical bonds in a crystal to indentation.” 2
Although Cco-C8 has a Vickers hardness slightly below that of diamond, the scientists predict that Cco-C8 should be hard enough to scratch and crack diamond. As Tian explains, this is because Cco-C8’s compressive strength is higher than diamond’s shear strength.
“The mechanical strength or ideal strength of a material depends on the loading modes of tensile, shear and compression,” he said. “For example, both the tensile strength and shear strength of diamond are about 90 GPa, while its compressive strength is up to 223 GPa. If forced into the surface of a single diamond crystal, Cco-C8 as an indenter is mainly in a compressed state, the chemical bonds of diamond below the indenter withstand compressive deformation, and the bonds around the indenter withstand shear deformation. Although Cco-C8 has slightly lower hardness than diamond, the compressive strength of Cco-C8 should be much higher than the shear strength of diamond. When the stress in the shear deformation zone exceeds the shear strength of diamond, an indentation is formed. In other words, Cco-C8 is able to crack diamond.”
Cco-C8 may not be too difficult to synthesize in the future. The simulations showed that Cco-C8 is highly stable; the new carbon allotrope is energetically more favorable than almost all other theoretical carbon structures. Also, the simulations suggest that Cco-C8 can be synthesized by directly compressing carbon nanotube bundles in a similar way to synthesizing 3D C60 polymers.
In fact, Cco-C8 may have already been synthesized unknowingly. Previous experiments on the cold compression of carbon nanotube bundles yielded a new phase of carbon that was originally identified as P-62c. However, Tian and his coauthors think that the structure was more likely Cco-C8.
In addition, the researchers expect that other novel carbon materials with unique physical properties can be formed by similar compression techniques by using different sized nanotubes or other carbon structures. They plan to search for these materials in the future.
“First, we will use this strategy to design more novel carbon allotropes, especially conducting superhard carbons with partially sp2-hybridized C-C bonds (in the crystal structure of Cco-C8, each carbon atom is sp3-hybridized),” Tian said. “Second, we will try to synthesize these designed carbon materials using carbon nanotube bundles at high pressure and high temperature.”
He added that Cco-C8 could have applications in fields where diamond has been used as a superhard material. And if the CALYPSO search discovers conducting superhard carbon materials, they could have potential applications in electronic devices.
More information: 1Zhisheng Zhao, et al. “Novel Superhard Carbon: C-Centered Orthorhombic C8.” PRL 107, 215502 (2011). DOI: 10.1103/PhysRevLett.107.215502
2Bo Xu, Zhisheng Zhao, and Yongjun Tian. “Microscopic theory of hardness and design of novel superhard crystals.” (Invited Review), Int. J. Refract. Met. Hard. Mater. (under review)
Copyright 2011 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.