World's lightest material developed

A team of researchers from UC Irvine, HRL Laboratories and the California Institute of Technology have developed the world's lightest material – with a density of 0.9 mg / cc – about 100 times lighter than Styrofoam. Their findings appear in the Nov. 18 issue of Science.
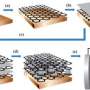
The new material redefines the limits of lightweight materials because of its unique "micro-lattice" cellular architecture. The researchers were able to make a material that consists of 99.99 percent air by designing the 0.01 percent solid at the nanometer, micron and millimeter scales. "The trick is to fabricate a lattice of interconnected hollow tubes with a wall thickness 1,000 times thinner than a human hair," said lead author Dr. Tobias Schaedler of HRL.
The material's architecture allows unprecedented mechanical behavior for a metal, including complete recovery from compression exceeding 50 percent strain and extraordinarily high energy absorption.
"Materials actually get stronger as the dimensions are reduced to the nanoscale," explained UCI mechanical and aerospace engineer Lorenzo Valdevit, UCI's principal investigator on the project. "Combine this with the possibility of tailoring the architecture of the micro-lattice and you have a unique cellular material."
Developed for the Defense Advanced Research Projects Agency, the novel material could be used for battery electrodes and acoustic, vibration or shock energy absorption.
William Carter, manager of the architected materials group at HRL, compared the new material to larger, more familiar edifices: "Modern buildings, exemplified by the Eiffel Tower or the Golden Gate Bridge, are incredibly light and weight-efficient by virtue of their architecture. We are revolutionizing lightweight materials by bringing this concept to the nano and micro scales."
More information: Ultralight Metallic Microlattices, Science 18 November 2011: Vol. 334 no. 6058 pp. 962-965. DOI: 10.1126/science.1211649
ABSTRACT
Ultralight (<10 milligrams per cubic centimeter) cellular materials are desirable for thermal insulation; battery electrodes; catalyst supports; and acoustic, vibration, or shock energy damping. We present ultralight materials based on periodic hollow-tube microlattices. These materials are fabricated by starting with a template formed by self-propagating photopolymer waveguide prototyping, coating the template by electroless nickel plating, and subsequently etching away the template. The resulting metallic microlattices exhibit densities ρ ≥ 0.9 milligram per cubic centimeter, complete recovery after compression exceeding 50% strain, and energy absorption similar to elastomers. Young’s modulus E scales with density as E ~ ρ2, in contrast to the E ~ ρ3 scaling observed for ultralight aerogels and carbon nanotube foams with stochastic architecture. We attribute these properties to structural hierarchy at the nanometer, micrometer, and millimeter scales.
Provided by University of California - Irvine