Molecules 'light up' Alzheimer's roots: Light-switching complex attaches itself to amyloid proteins
(PhysOrg.com) -- A breakthrough in sensing at Rice University could make finding signs of Alzheimer's disease nearly as simple as switching on a light.
The technique reported in the Journal of the American Chemical Society should help researchers design better medications to treat the devastating disease.
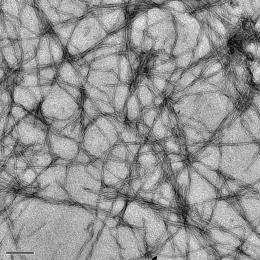
The lab of Rice bioengineer Angel Martí is testing metallic molecules that naturally attach themselves to a collection of beta amyloid proteins called fibrils, which form plaques in the brains of Alzheimer's sufferers. When the molecules, complexes of dipyridophenazine ruthenium, latch onto amyloid fibrils, their photoluminescence increases 50-fold.
The large increase in fluorescence may be an alternative to molecules currently used to study amyloid fibrils, which researchers believe form when misfolded proteins begin to aggregate. Researchers use changes in fluorescence to characterize the protein transition from disordered monomers to aggregated structures.
Nathan Cook, a former Houston high school teacher and now a Rice graduate student and lead author of the new paper, began studying beta amyloids when he joined Martí's lab after taking a Nanotechnology for Teachers course taught by Rice Dean of Undergraduates and Professor of Chemistry John Hutchinson. Cook's goal was to find a way to dissolve amyloid fibrils in Alzheimer's patients.
But the Colorado native's research led him down a different path when he realized the ruthenium complexes, the subject of much study in Martí's group, had a distinctive ability to luminesce when combined in a solution with amyloid fibrils.
Such fibrils are simple to make in the lab, he said. Molecules of beta amyloid naturally aggregate in a solution, as they appear to do in the brain. Ruthenium-based molecules added to the amyloid monomers do not fluoresce, Cook said. But once the amyloids begin to aggregate into fibrils that resemble "microscopic strands of spaghetti," hydrophobic parts of the metal complex are naturally drawn to them. "The microenvironment around the aggregated peptide changes and flips the switch" that allows the metallic complexes to light up when excited by a spectroscope, he said.
Thioflavin T (ThT) dyes are the standard sensors for detecting amyloid fibrils and work much the same way, Marti said. But ThT has a disadvantage because it fluoresces when excited at 440 nanometers and emits light at 480 nanometers -- a 40-nanometer window.
That gap between excitation and emission wavelengths is known as the Stokes shift. "In the case of our metal complexes, the Stokes is 180 nanometers," said Martí, an assistant professor of chemistry and bioengineering. "We excite at 440 and detect in almost the near-infrared range, at 620 nanometers.
"That's an advantage when we want to screen drugs to retard the growth of amyloid fibrils," he said. "Some of these drugs are also fluorescent and can obscure the fluorescence of ThT, making assays unreliable."
Cook also exploited the metallic's long-lived fluorescence by "time gating" spectroscopic assays. "We specifically took the values only from 300 to 700 nanoseconds after excitation," he said. "At that point, all of the fluorescent media have pretty much disappeared, except for ours. The exciting part of this experiment is that traditional probes primarily measure fluorescence in two dimensions: intensity and wavelength. We have demonstrated that we can add a third dimension -- time -- to enhance the resolution of a fluorescent assay."
The researchers said their complexes could be fitting partners in a new technique called fluorescence lifetime imaging microscopy, which discriminates microenvironments based on the length of a particle's fluorescence rather than its wavelength.
Cook's goal remains the same: to treat Alzheimer's -- and possibly such other diseases as Parkinson's -- through the technique. He sees a path forward that may combine the ruthenium complex's ability to target fibrils and other molecules' potential to dissolve them in the brain.
"That's something we are actively trying to target," Martí said.
More information: pubs.acs.org/doi/abs/10.1021/ja204656r
Provided by Rice University















