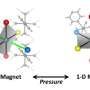
Tuning the collective properties of artificial nanoparticle supercrystals
Precise ordering in two-dimensional (2-D) and three-dimensional (3-D) superlattices formed by the self-assembly of individual nanocrystals (NCs) allows for control of the magnetic, optical, and electronic coupling between the individual NCs. This control can lead to useful collective properties such as vibrational coherence, reversible metal-to-insulator transitions, enhanced conductivity, spin-dependent electron transport, enhanced ferro- and ferrimagnetism, tunable magnetotransport, and efficient charge transport. These properties have many potential applications in solar cells, field-effect transistors, light-emitting devices, photodetectors, and photoconductors.
Due to precise positioning of the NCs within a 3-D superlattice, such systems are frequently referred to as “supercrystals” (SCs) in analogy to crystals built of atoms. But unlike the atomic crystals, SCs offer the flexibility of tuning the interparticle distance due to presence of the “soft” shell of organic ligands that can be used to control collective properties in such structures. Structural stability and compressibility are fundamental characteristics of any 3-D system.
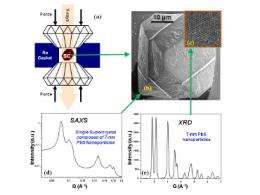
A team of researchers from the Argonne National Laboratory Center for Nanoscale Materials, the X-ray Science Division at the Argonne Advanced Photon Source (APS), University of Chicago’s GeoSoilEnviroCARS, which operates Sector 13 at the APS, and Northwestern University have reported on the first combined quasi-hydrostatic, high-pressure, small-angle x-ray scattering (SAXS) and micro x-ray diffraction (XRD) studies on individual faceted, 3-D supercrystals self-assembled from colloidal 7.0-nm PbS nanocrystals. Combining the SAXS and XRD techniques allowed for precise evaluation of the interparticle spacing during the pressure cycling since the volume change of the individual NCs was taken into account. Neon was used as a pressure transmitting media to avoid the possibility of the leaching of organic ligands from the surface of the NCs and losing the structural integrity of the SCs due to sintering. Diamond anvil cell (DAC) SAXS experiments in the pressure range from ambient to 12.5 GPa, performed at X-ray Science Division x-ray beamline 12-ID-C at the APS, revealed nearly perfect structural stability of the SCs, with fcc organization of the NCs. The XRD experiments, which were carried out at GeoSoilEnviroCARS x-ray beamline 13-ID-D at the APS, demonstrated that NCs have strong preferential orientation of individual NCs in SCs up to ~55 GPa that is preserved during pressure cycling.
The mechanical properties of the individual NCs, their SCs, and the ligand matrix were analyzed using the equation of states derived from the compression data produced by SAXS and XRD. Ambient pressure bulk modulus of the SCs was calculated to be ~5 GPa during compression and ~14.5 GPa during the release cycle, respectively. NCs were found to undergo first-order phase transition above 8 GPa, and the transformation proceeds through a single nucleation event (within a pressure range of 8.1-9.2 GPa) during the first transition, and heterogeneous nucleation during the second transformation from the intermediate phase (that is not yet identified) to CsCl structure. A bulk modulus for the ligand matrix of ~2.2-2.95 GPa is an order of magnitude greater than that observed from nanoindentation study.
The high structural stability of the SCs and the ability to tune the interparticle spacing seem to offer the promise of further manipulation of the collective properties of self-organized artificial solids including the structures that consisted of NCs transformed at high pressures into a different phase. Combining high-pressure XRD and SAXS provides unique opportunities to obtain direct information about the mechanical properties of individual building blocks and their hierarchical architectures.
More information: Paul Podsiadlo, et al. “High-Pressure Structural Stability and Elasticity of Supercrystals Self-Assembled from Nanocrystals,” Nanolett. Article ASAP, published online ahead of print, December 22, 2010
Provided by Argonne National Laboratory