February 17, 2011 weblog
C60 could form a new kind of gel
(PhysOrg.com) -- C60, the spherical carbon molecule also known as a buckminsterfullerene, has intrigued scientists for its unique properties and potential applications in nanotechnology and electronics. Now scientists have found that C60 may have another unusual property: it may take the form of a one-component gel under certain conditions. To date, all known gels consist of two components: an evenly distributed substance (a colloid) and a substance that dissolves the colloid (a solvent).
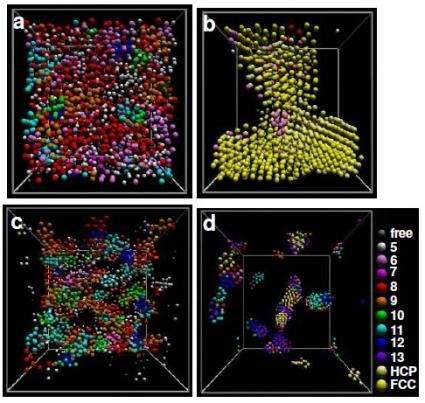
Scientists have previously discovered that C60 can take the form of different phases of matter, including solids and liquids. Here, chemists Patrick Royall from the University of Bristol and Stephen Williams from the Australian National University found that C60 can theoretically exist in a dense liquid phase containing clusters, which bind together to form a gel structure, specifically a "spinodal" gel. The gel is made entirely of carbon.
In their study, the scientists performed computer simulations showing that C60 can form a gel at moderately high temperatures and very high quench rates. The simulations showed that C60 gels form in about 10 nanoseconds and are stable at room temperature for at least 100 nanoseconds, which is the maximum time that the simulations were run. Although the gel showed some coarsening, the scientists predict that it would remain stable for more than 100 nanoseconds. Eventually, however, the gel would separate into a crystal and a gas.
As far as experimentally demonstrating the C60 gel, the scientists predict that it will be a challenge, largely due to the extremely high quench rates required, which are not currently experimentally feasible. However, they may investigate creative ways to lower the quench rate, or try to use larger fullerenes such as C540, which may also become a carbon gel. In any case, the potential existence of a one-component gel could lead to an overall better understanding of the nature of gels.
More information:
C. Patrick Royall and Stephen R. Williams. "C60: the first one-component gel?" arXiv:1102.2959v1 [cond-mat.soft]
via: The Physics arXiv Blog
© 2010 PhysOrg.com