Communication pathways within proteins may yield new drug targets to stop superbugs
A School of Science at Indiana University-Purdue University Indianapolis biophysicist has developed a new method to identify communication pathways connecting distant regions within proteins.
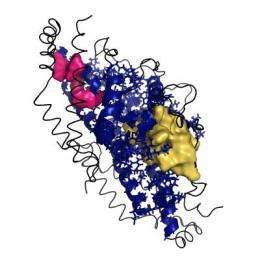
With this tool, Andrew J. Rader, Ph.D., assistant professor of physics, has identified a mechanism for cooperative behavior within an entire molecule, a finding that suggests that in the future it may be possible to design drugs that target anywhere along the length of a molecule's communication pathway rather than only in a single location as they do today. The discovery holds promise for increasing the likelihood of therapeutic success.
The study, "Correlating Allostery with Rigidity" is published in the current issue of Molecular BioSystems, a journal of the Royal Society of Chemistry.
Microorganisms frequently contain enzymes, protein molecules that carry out most of the important functions of cells, not present in human cells. Blocking these enzymes can stop or kill a harmful invader.
Drugs are often developed to block or restrict the function of such enzymes, thereby treating the underlying infectious disease they convey. These drugs often target specific chemical sites on bacterial or viral enzymes, and alter the enzymes so they no longer function. Unfortunately, microorganisms can evolve enzymes that are impervious to these drugs, resulting in drug resistant organisms.
"With the growth of drug resistant organisms, it is increasingly important that we gain a better understanding of what makes enzymes within cellular proteins do what they do, so that we can develop alternative approaches to targeting these proteins, shutting down enzymes and killing these superbugs," said Rader, first author of the study.
He has found that the "poking" of one spot on the rigid pathway connecting regions within proteins produces communication along the entire pathway, indicating that drugs could be targeted to multiple locations on the pathways that had not developed drug resistance and could travel to where needed. His new method identified more than twice as many communication pathways as previous studies.
To use the analogy of a railroad track, dislocating a single rail, anywhere on the track, effects the entire track as trains cannot travel from one end to the other due to the rail that is out of alignment. Returning the rail to its proper location makes the entire track function normally. In the case of the rigid pathways within proteins, affecting a single chemical locus on the pathway affects the entire pathway.
"We now see in these rigid pathways that we can effect something at a distance. This holds great potential for drug targeting. We can do something at one site on the pathway, where drug resistance is not an issue, and it will affect another, perhaps turning an enzyme off and eliminating drug resistance. It's too early to say whether we can successfully counter tuberculosis, Methicillin-resistant Staphylococcus aureus [MRSA] and others of the growing number of multidrug resistant organisms this way, but it's a promising approach well worth further exploration," said Rader.
More information: Mol. BioSyst., 2011, 7, 464-471
Provided by Indiana University