Carbonic acid: now isolated in the gas phase and examined spectroscopically
(PhysOrg.com) -- Until now, it has stubbornly defied all attempts at detection: according to current textbooks, gas-phase carbonic acid should not exist at all, because it immediately decomposes into water and carbon dioxide –making it detectible only as a trace component.
A team led by Hinrich Grothe at the TU Vienna and Thomas Loerting at the University of Innsburck has now finally found proof to the contrary. As the Austrian researchers report in the journal Angewandte Chemie, they were able to isolate carbonic acid and gather spectroscopic data.
Carbonated beverages contain carbon dioxide. They also contain trace amounts of a molecule that was long thought to be too unstable to exist: carbonic acid (H2CO3). It is now known that carbonic acid is indeed present in drinks, though at very, very low concentrations. Until recently, the molecule has resisted all attempts at isolation and direct detection. However, a few scientists have been able to produce carbonic acid in the solid state. It is also assumed to be present in cirrus clouds in Earth’s atmosphere and in space.
The Austrian researchers have now demonstrated that carbonic acid can exist in the gas phase and that it is stable at temperatures up to –30 °C. For these experiments, solid carbonic acid was formed by means of acid-base reactions at very low temperatures and then warmed to –30 °C. The evaporating molecules were trapped in a matrix of the noble gas argon and then immediately cooled again. This resulted in a kind of frozen “image” of the gas-phase carbonic acid, which the researchers were able to study by infrared spectrometry.
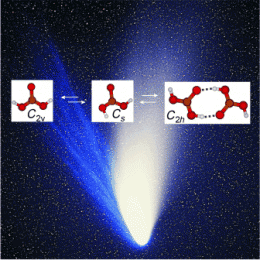
The spectra showed that gas-phase carbonic acid exists in three different forms. The scientists found two monomers that differ in their conformation—the spatial arrangement of their atoms—as well as a dimer made from two molecules bound through hydrogen bonds.
The resulting detailed spectrometric data are of great interest to astronomers, because they could make it easier to detect gas-phase carbonic acid in space, where it is thought to be present in the tails of comets and on Mars.
More information: Hinrich Grothe, Spectroscopic Observation of Matrix-Isolated Carbonic Acid, Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201004729
Provided by Wiley