Blocking the critical structure that lets cancer cells move -- their feet
Scientists now know that some cancer cells spread, or metastasize, throughout the body the old-fashioned way -- by using their feet. But researchers at Duke Cancer Institute have discovered a way to short-circuit their travels by preventing the development of these feet, called invadopodia. This discovery is even more important because preventing the development of these "feet" also eliminates the action of proteins present in the feet that burn through intact tissue and let cancer cells enter new cells.
The results could yield a treatment to prevent the spread of cancer, which would be taken in combination with a treatment that kills the cancer cells, said Ann Marie Pendergast, Ph.D., senior author and James B. Duke Professor of Pharmacology and Cancer Biology at Duke. "A combination like this would be more effective than either treatment given alone."
"This is the first time anyone has identified the Abl family of protein kinases (comprising two proteins, Abl and Arg) as critical regulators of invadopodia structures," Pendergast said. "This has never been seen before."
The study was published in the Journal of Biological Chemistry on Dec. 17.
The team found that the Abl and Arg kinases are required not only for the formation and function of the invadopodia, but also that these kinases are found within these structures. "Thus, if we can find a way to block the kinases, we'll find a way to keep the feet from forming correctly and will keep the cells from moving," Pendergast said.
The researchers also made a new connection between these Abl and Arg kinases and the regulation of a Matrix Metalloproteinase (MMP) that is very important in cancer invasion and metastasis. "When you lose the functions of the Abl and Arg kinases, you also lose the function of the MMP proteins, which 'chew' through the matrix surrounding cells and tissues," Pendergast said. The MMP proteins can create openings for cancerous cells to escape through on their way to becoming a metastasis, she said.
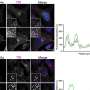
The studies began because the researchers knew that Abl kinases can directly connect with actin, a filament-like protein that cells use to move. These kinases also seem to target a number of actin-regulatory proteins that are found in invadopodia, "so we thought it might be interesting to see what would happen if we blocked the activity of these kinases," Pendergast said. "We expected a mild effect, but were surprised by the striking effect we saw."
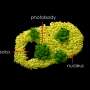
Using fluorescent proteins for imaging purposes, the team observed that when the kinase activity was blocked, the cancer cell "feet" then disappeared as well.
Pendergast said the pharmacologic agents used to block the Abl kinases are FDA-approved for use in leukemia (imatinib), which means it may not be hard to win their approval for new applications.
Provided by Duke University Medical Center