Alternating stacks of planar cations and dipyrrole-containing anions provides concept for new materials

(PhysOrg.com) -- Pyrroles, which are rings containing one nitrogen and four carbon atoms, are essential components of our red hemoglobin as well as the green chlorophyll in plants. Japanese researchers led by Hiromitsu Maeda at Risumeikan University have now also used this molecular motif in the construction of new nanostructured materials: They combined planar pyrrole-containing negatively charged complexes with similarly planar, positively charged organic ions.
As the scientists report in the journal Angewandte Chemie, they were able to produce fibers and soft materials, such as supramolecular gels and liquid crystals.
Salts consist of cations and anions—positively and negatively charged particles. Most salts organize themselves into ordered crystals that are held together through the electrostatic attraction between the oppositely charged ions. However, there are also ionic liquids, which are salts that exist as melts at room temperature. The size and geometry of the ions involved prevent the formation of a strong crystal lattice.
Ionic liquid crystals are another interesting class of materials. Liquid crystals are fluid like a liquid, though the particles in them are arranged in an ordered state. In addition, there are other materials that are more organized but whose components maintain a certain degree of mobility. These are of interest for the development of ferroelectric memory devices.
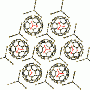
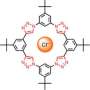
The Japanese researchers selected planar ions to build up self-organized materials in which the charged components are stacked in an alternating fashion. The first component is a planar complex made from a small inorganic ion and an organic receptor (receptor–anion complex). The critical structural element of the receptor contains two pyrroles bound into what is known as a π-conjugated environment. This means that some of the electrons are freely mobile as an “electron cloud” over a large area of the molecule. The ligand surrounds the anion on three sides.
The second component is a disk-shaped organic cation made from an aromatic ring system, which also has an electron cloud. Because of the electrostatic attraction between oppositely charged ions, and also attractive interactions between the electron clouds, these anions and cations always stack themselves into alternating columnar units.
Depending on the type of additional side-groups on the components, the columns organize into various structures, such as fibers, supramolecular gels, or liquid crystals. Such alternating stacks of oppositely charged components (charge-by-charge assembly) has proven to be a successful concept for the production of novel materials from organic ions.
More information: Hiromitsu Maeda, Oriented Salts: Dimension-Controlled Assemblies from Planar Receptor–Anion Complexes, Angewandte Chemie International Edition 2010, 49, No. 52, 10079–10083, dx.doi.org/10.1002/anie.201006356
Provided by Wiley