Hydrogen generation without the carbon footprint
A greener, less expensive method to produce hydrogen for fuel may eventually be possible with the help of water, solar energy and nanotube diodes that use the entire spectrum of the sun's energy, according to Penn State researchers.
"Other researchers have developed ways to produce hydrogen with mind-boggling efficiency, but their approaches are very high cost," says Craig A. Grimes, professor of electrical engineering. "We are working toward something that is cost effective."
Currently, the steam reforming of natural gas produces most of our hydrogen. As a fuel source, this produces two problems. The process uses natural gas and so does not reduce reliance on fossil fuels; and, because one byproduct is carbon dioxide, the process contributes to the carbon dioxide in the atmosphere, the carbon footprint.
Grimes' process splits water into its two components, hydrogen and oxygen, and collects the products separately using commonly available titanium and copper. Splitting water for hydrogen production is an old and proven method, but in its conventional form, it requires previously generated electricity. Photolysis of water solar splitting of water has also been explored, but is not a commercial method yet.
Grimes and his team produce hydrogen from solar energy, using two different groups of nanotubes in a photoelectrochemical diode. They report in the July issue of Nano Letters that using incident sunlight, "such photocorrosion-stable diodes generate a photocurrent of approximately 0.25 milliampere per centimeter square, at a photoconversion efficiency of 0.30 percent."
"It seems that nanotube geometry is the best geometry for production of hydrogen from photolysis of water," says Grimes
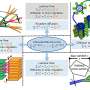
In Grimes' photoelectrochemical diode, one side is a nanotube array of electron donor material – n-type material – titanium dioxide, and the other is a nanotube array that has holes that accept electrons - p-type material – cuprous oxide titanium dioxide mixture. P and n-type materials are common in the semiconductor industry. Grimes has been making n-type nanotube arrays from titanium by sputtering titanium onto a surface, anodizing the titanium with electricity to form titanium dioxide and then annealing the material to form the nanotubes used in other solar applications. He makes the cuprous oxide titanium dioxide nanotube array in the same way and can alter the proportions of each metal.
While titanium dioxide is very absorbing in the ultraviolet portion of the sun's spectrum, many p-type materials are unstable in sunlight and damaged by ultraviolet light, they photo-corrode. To solve this problem, the researchers made the titanium dioxide side of the diode transparent to visible light by adding iron and exposed this side of the diode to natural sunlight. The titanium dioxide nanotubes soak up the ultraviolet between 300 and 400 nanometers. The light then passes to the copper titanium side of the diode where visible light from 400 to 885 nanometers is used, covering the light spectrum.
The photoelectrochemical diodes function the same way that green leaves do, only not quite as well. They convert the energy from the sun into electrical energy that then breaks up water molecules. The titanium dioxide side of the diode produces oxygen and the copper titanium side produces hydrogen.
Although 0.30 percent efficiency is low, Grimes notes that this is just a first go and that the device can be readily optimized.
"These devices are inexpensive and because they are photo-stable could last for years," says Grimes. "I believe that efficiencies of 5 to 10 percent are reasonable."
Grimes is now working with an electroplating method of manufacturing the nanotubes, which will be faster and easier.
Provided by Penn State