Croc's deadly last meal in Ancient Egypt unearthed
Scientists have used state of the art 3D imaging technology to piece together the life—and probable death—of a 2.2 meter-long crocodile mummified by the ancient Egyptians.

Scientists have used state of the art 3D imaging technology to piece together the life—and probable death—of a 2.2 meter-long crocodile mummified by the ancient Egyptians.
Archaeology
Jul 12, 2024
0
53

Clean drinking water is a basic demand for our health and well-being. However, as the global population grows, achieving this for all communities worldwide becomes more challenging.
Polymers
Jul 29, 2024
0
33

A global research team led by Texas Engineers has developed a way to blast the molecules in plastics and other materials with a laser to break them down into their smallest parts for future reuse.
Nanophysics
Jul 9, 2024
1
139

A research team led by Director Jo Moon-Ho of the Center for Van der Waals Quantum Solids within the Institute for Basic Science (IBS) has implemented a novel method to achieve epitaxial growth of 1D metallic materials with ...
Nanomaterials
Jul 3, 2024
0
354

An exoplanet infamous for its deadly weather has been hiding another bizarre feature—it reeks of rotten eggs, according to a new Johns Hopkins University study of data from the James Webb Space Telescope.
Planetary Sciences
Jul 8, 2024
0
97

A surprising discovery about the evolution of our galaxy using data from the Gaia mission found a large number of ancient stars on orbits similar to that of our sun. They formed the Milky Way's thin disk less than 1 billion ...
Astronomy
Jul 31, 2024
4
194

The president of SUNY Polytechnic Institute (SUNY Poly), Dr. Winston "Wole" Soboyejo, and postdoctoral researcher Dr. Tabiri Kwayie Asumadu have published a paper titled, "Robust Macroscale Superlubricity on Carbon-Coated ...
Analytical Chemistry
Jul 15, 2024
0
176

As Independence Day approaches, Utahns are preparing to celebrate the nation's birth with dazzling displays of light and color. However, a new BYU study published in Applied Geochemistry warns that these festivities come ...
Environment
Jul 2, 2024
1
177

A new study led by researchers in Canada introduces a novel process for the extraction and separation of metals from spent alkaline batteries, offering a promising solution for efficient recycling of critical materials.
Materials Science
Jul 3, 2024
0
2
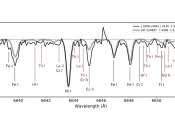
Using the Gran Telescopio Canarias (GTC), astronomers from the Chinese Academy of Sciences (CAS) and elsewhere have discovered a new extremely r-process-enhanced star in the Milky Way's thin disk. The finding was reported ...
In chemistry, a metal (Greek: Metallo, Μέταλλο) is an element, compound, or alloy characterized by high electrical conductivity. In a metal, atoms readily lose electrons to form positive ions (cations); those ions are surrounded by delocalized electrons, which are responsible for the conductivity. The thus produced solid is held by electrostatic interactions between the ions and the electron cloud, which are called metallic bonds.
This text uses material from Wikipedia, licensed under CC BY-SA