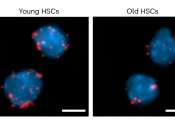
Study uncovers the secret of long-lived stem cells
Nothing lives forever, but compared to other cells in the body, hematopoietic stem cells (HSCs) are remarkably long-lived. HSCs are blood-forming cells—they give rise to rapidly dividing progenitor cells, which in turn ...