This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Glitch in protein synthesis could affect tumor growth
During protein synthesis, or translation, genetic information transcribed in the cell's mRNA directs the stringing together of amino acids—the building blocks of proteins. As the translation machinery carouses along the string of nucleotides that make up the mRNA, it recognizes them in groups of three, called codons. Each codon corresponds to a specific amino acid.
Certain codons tell the translation machinery where to start and stop. But sometimes, like a rogue driver skipping a red light, the machinery skips the stop codon and ends up making a longer protein than planned. This is called stop codon readthrough.
"Because of that extra appendage, the protein can have a different localization, a different stability, it can even have a different function," explains Sandeep Eswarappa, Associate Professor at the Department of Biochemistry (BC), Indian Institute of Science (IISc).
In a study published in the Journal of Cell Science, Eswarappa's team zeroed in on a gene that codes for a protein called FEM1B. They show how FEM1B mRNA readthrough plays a key role in the cell cycle, with implications for cancer cell proliferation and tumor growth.
The FEM1B protein is part of a complex that marks other proteins for degradation. Its job is to make sure that the right proteins are marked. The complex targets key proteins involved in many cellular processes—one of them being the cell cycle, to keep a check on how much cells proliferate.

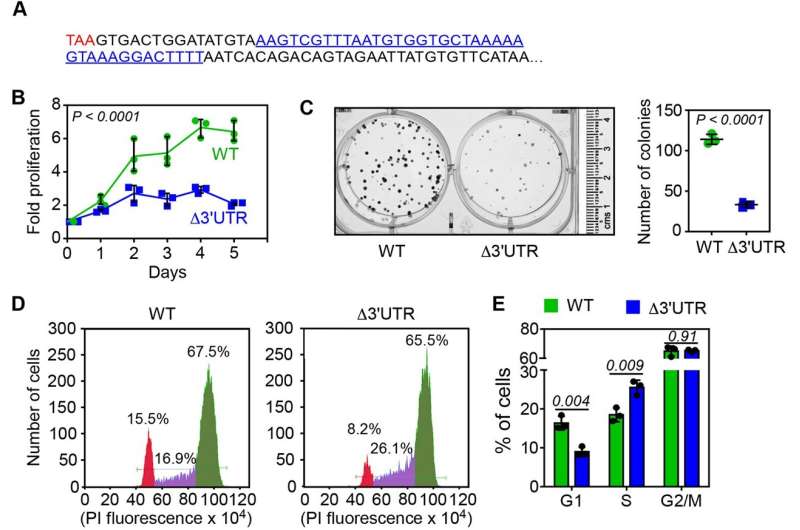
In the study, the researchers found that stop codon readthrough causes the translation machinery to make a longer and more unstable version of FEM1B. Ironically, this marks FEM1B itself for degradation, leading to reduced levels of the protein. The team found that a specific nucleotide sequence at the tail end of the FEM1B gene directs this readthrough.
The team then became curious about how this would affect cancer cell growth—a hallmark of the deadly disease is the uncontrolled proliferation of cells. In lab-grown cancer cell lines, they deployed the CRISPR-Cas9 system—commonly used "molecular scissors"—to snip off the sequence driving the readthrough from the FEM1B gene.
Preventing readthrough led to increased levels of the FEM1B protein—and therefore increased degradation of target proteins—and a delayed cell cycle. This caused the cancer cells to proliferate less.
"This was surprising, as it brought a clinical relevance to this study," says lead author Md Noor Akhtar, Integrated Ph.D. student in Eswarappa's lab. The team then injected the readthrough-defective cancer cell lines in a mice model and found that the consequent tumors grew more slowly.
Delving into publicly available datasets of human cancer patients, the researchers also discovered that a higher expression of the FEM1B gene correlated with an increased probability of survival, which was consistent with their hypothesis.
When they looked at the evolution of the FEM1B gene, they realized that the readthrough process seems to be found specifically in humans and chimpanzees. They think that this glitch might have occurred due to the insertion of a single nucleotide downstream of the first stop codon—a tiny change that happened about 10 million years ago, when humans and chimpanzees branched off from other primates.
This could be one of the factors that contributed to increased susceptibility for cancer in humans compared to other primates, the authors speculate.
Eswarappa's team is still puzzled by how exactly the stop codon readthrough happens. They are keen on nailing down the molecular machinery involved, which will help make any therapeutic approach more specific. He adds, "If we know the mechanism, we can target and regulate the readthrough process, which in turn might help in controlling the tumor progression."
More information: Md Noor Akhtar et al, Hominini-specific regulation of the cell cycle by stop codon readthrough of FEM1B, Journal of Cell Science (2024). DOI: 10.1242/jcs.261921
Journal information: Journal of Cell Science
Provided by Indian Institute of Science