This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
High-speed atomic force microscopy reveals dynamic behavior of brain receptors

Researchers at the Nano Life Science Institute (WPI-NanoLSI), Kanazawa University, used high-speed atomic force microscopy to observe dynamic changes in AMPA receptors, which are vital for brain communication. Their findings, published in ACS Nano, reveal how these receptors adapt during signal transmission and suggest potential targets for neurological therapies.
This study, led by Mikihiro Shibata, delves into the complex behavior of AMPA receptors (AMPARs), which are crucial for communication between nerve cells in the brain.
AMPARs are responsible for fast excitatory neurotransmission, a process crucial for learning, memory, and overall cognitive function. The research particularly focuses on the GluA2 subunit of AMPARs, a key component in transmitting signals at synapses, the junctions where neurons connect.
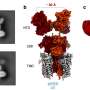
The team employed an advanced imaging technique known as high-speed atomic force microscopy (HS-AFM) to observe the real-time behavior of the N-terminal domain (NTD) in the GluA2 subunit. The NTD is the starting segment of the protein, playing a critical role in how AMPARs function and cluster at synapses.
The study also examined how the GluA2 subunit interacts with TARP γ2, a regulatory protein that fine-tunes the receptor's response to signals.
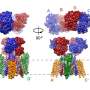
One of the key findings is the behavior of the NTD in different states: resting, activated, and desensitized. The researchers discovered that in the activated state, the NTD dimers—pairs of NTDs—can split into single units or monomers. This process, known as subunit exchange, allows parts of one receptor to swap with another, potentially altering the receptor's function.
This novel observation was supported by molecular dynamics simulations, which showed that these monomeric states are stable in their lipid environment, providing a potential mechanism for receptor adaptability and diversity.
In the desensitized state, where the receptor becomes less responsive to signals, the NTD dimers separate, but their movement is more restricted compared to the activated state. This desensitization helps protect nerve cells from overstimulation, which could lead to cellular damage.
The study's insights into the structural changes of the NTDs in different functional states highlight the dynamic nature of AMPARs and their ability to adapt to various conditions within the synaptic environment.
The research also sheds light on the role of neuronal pentraxin 1 (NP1), a protein that aids in the clustering of AMPARs at synapses. NP1 forms a ring-shaped structure that binds to the tips of the NTDs, potentially facilitating the gathering of multiple AMPARs into clusters.
This clustering is essential for efficient synaptic transmission, as it brings receptors closer together, allowing for more effective signaling between neurons. By linking multiple receptors, NP1 enhances the strength and reliability of the synaptic connection, contributing to the overall efficiency of neural communication.
The study's findings contribute significantly to our understanding of how AMPARs function and adapt during neurotransmission. By revealing the dynamic structural changes in the NTDs and highlighting the role of NP1 in receptor clustering, the research offers new insights into the molecular processes that underlie synaptic plasticity—the ability of synapses to strengthen or weaken over time, which is essential for learning and memory.
These discoveries could have important implications for developing treatments for neurological disorders where AMPAR function is disrupted, such as in epilepsy, Alzheimer's disease, and other cognitive impairments.
As the authors conclude, "Our research reveals the dynamic structural changes that occur within AMPA receptors, underscoring their remarkable adaptability. Understanding these mechanisms not only deepens our knowledge of brain function but also opens new avenues for therapeutic interventions targeting synaptic transmission and plasticity."
More information: Ayumi Sumino et al, High-Speed Atomic Force Microscopy Reveals Fluctuations and Dimer Splitting of the N-Terminal Domain of GluA2 Ionotropic Glutamate Receptor-Auxiliary Subunit Complex, ACS Nano (2024). DOI: 10.1021/acsnano.4c06295
Journal information: ACS Nano
Provided by Nano Life Science Institute (NanoLSI), Kanazawa University