This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Getting trapped in freshwater ice changes microplastics' sink-or-swim tendencies
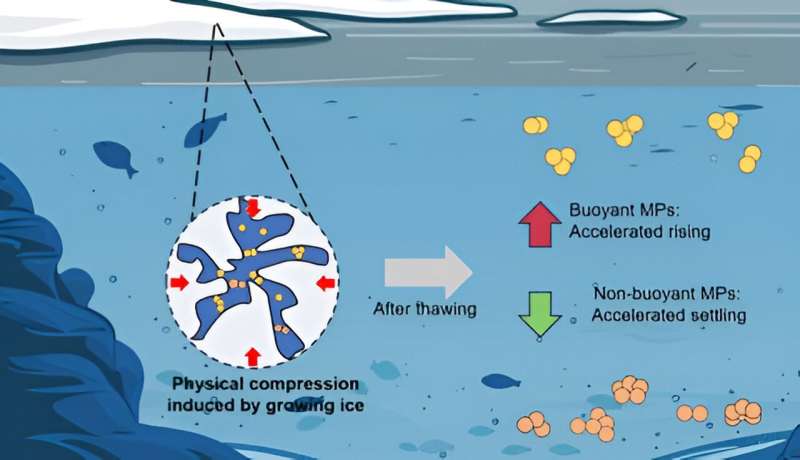
Most bodies of water contain minute plastic particles that get trapped at the surface when ice forms. But when the ice melts, the microplastics have changed. According to research recently published in Environmental Science & Technology, the thawed particles might be larger and sink or float faster, depending on the polymer type. The study's authors say freezing could alter the environmental impact of microplastics, including their accumulation in freshwater lake or river sediments.
The polymers that produce microplastics have different behaviors in the environment. For instance, polyethylene (PE) is less dense than water and floats, whereas polyurethane (PU) and polytetrafluorethylene (PTFE) are denser than water and sink toward lake beds and seafloors. Buoyancy, as well as particle size, salinity and other environmental conditions, affect whether the microplastics stay at the water's surface or get buried in sediment. To understand how freezing impacts PE, PU and PTFE particles, Chunjiang An and colleagues conducted laboratory experiments with fresh and saltwater.
An and the research group started with particles of each polymer (between 6 and 10 micrometers) in separate solutions, ranging from freshwater to the salinity levels in seawater. The team froze the samples for 24 hours and then completely thawed them. In freshwater, all three polymers' particles increased in size after freezing compared to a control kept cool for 24 hours. Specifically, they observed a greater change (46%) in PE, a polymer that repels water, than the increase (9%) in PU, a polymer that attracts water, which—the researchers suggest—causes particles to disperse more after freezing.
However, as salt levels increased in the samples, freezing did not impact any polymer's particle size compared to the cool-temperature controls. The researchers hypothesize that brine channels within the ice structures give trapped particles spaces to avoid compressing and joining to form larger clumps.
Next, the researchers investigated the forces behind the particle movements they observed in the experiments, where more PTFE and PU microplastics settled at the bottom of the beakers after the freeze-thaw cycle than before. Using calculations of the balance between gravitational, buoyant and drag forces on the particles, they estimated that particles would rise (PE) or sink (PFTE and PU) in water faster after freezing and thawing from ice because of the increased buoyancy force on the particles.
The researchers acknowledge that the freezing period used in this study is shorter than in natural environments, where freezing can last several months or years. Therefore, they say this study provides a foundational examination of the fate of various polymer particles when released from ice in cold regions, and indicates that even rapid freezing could accelerate the amount of microplastics settling in sediments.
More information: Zhikun Chen et al, Revealing the Freezing-Induced Alteration in Microplastic Behavior and Its Implication for the Microplastics Released from Seasonal Ice, Environmental Science & Technology (2024). DOI: 10.1021/acs.est.4c05322
Journal information: Environmental Science & Technology
Provided by American Chemical Society