This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New approach expands quantification of nutrient exchange in plant tissues, the rhizosphere and soil
Organic carbon in soil is linked to enhanced plant growth and to improved subsurface biodiversity, and it is a potential sink for atmospheric carbon dioxide (CO2). Yet, injection of organic carbon into soil through various root processes is typically focused on small spatial regions, which can confound attempts to quantify the carbon and correlate it with various microenvironments that exist around plant roots.
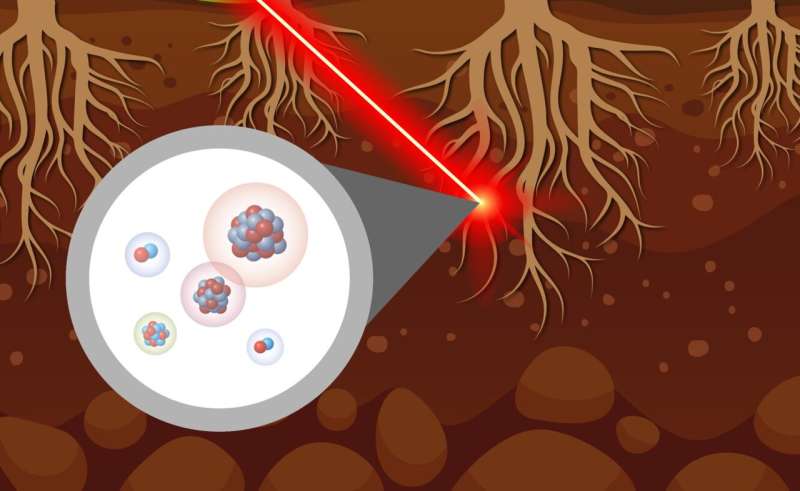
A multi-institutional team of researchers has developed and demonstrated a new approach to characterize carbon isotopic distribution within plant tissues, the rhizosphere, and soil. They began by exposing switchgrass plants to 13CO2 in a laboratory setting.
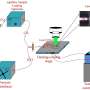
They leveraged a 13C tracer to selectively track photosynthetic materials as they were transferred through the plants' vascular tissues and exuded into the rhizosphere. Then, using laser ablation at the Environmental Molecular Sciences Laboratory, a Department of Energy Office of Science user facility, they rastered over the material and continuously ablated the sample and combusted the resulting material.
This sample-derived CO2 was pumped through a capillary absorption spectroscopy (CAS) fiber. Carefully balancing the vacuum strength helped the team optimize sample dwell time in the fiber to achieve suitable measurement precision before the sample exited the fiber.
The improved sampling density of the team's approach was made possible by employing the CAS isotope detector. The enhanced measurement sensitivity of CAS over conventional isotope ratio mass spectrometry was crucial to being able to run continuous analyses without needing to cryogenically trap the sample-derived CO2.
This approach avoids a significant time lag and thereby increases the richness of the stable isotope data to better address carbon cycling questions in plant tissues, the rhizosphere, and soil.
The findings are published in the journal Soil Biology and Biochemistry.
More information: Daniel M. Cleary et al, Laser Ablation-Capillary Absorption Spectroscopy: A novel approach for high throughput and increased spatial resolution measurements of δ13C in plant-soil systems, Soil Biology and Biochemistry (2023). DOI: 10.1016/j.soilbio.2023.109208
Provided by Environmental Molecular Sciences Laboratory