This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Study proposes large-scale biomanufacturing workflow to produce natural killer cells and extracellular vesicles
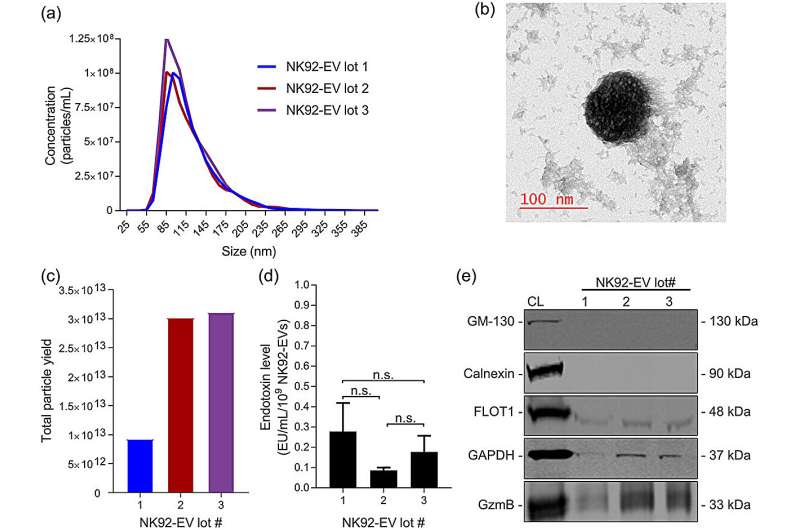
A team of uOttawa Faculty of Medicine researchers have developed a path to a biomanufacturing process that could potentially transform how Canada generates immunotherapeutic materials—specifically natural killer cells and extracellular vesicles (EVs)—to fuel tomorrow's novel cancer treatments.
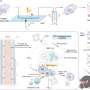
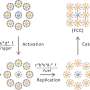
How does it work? Their proof-of-concept study focuses on using a hollow-fiber bioreactor—a type of high-density cell culture system that's bundled in a small cartridge containing thousands of semipermeable fibers. The team's method detailed in the Journal of Extracellular Vesicles would allow scientists to generate a continuous flow of cell-derived immunotherapeutics without compromising the materials' quality and anti-cancer characteristics.
This could significantly boost the feasibility of setting up cost-effective biomanufacturing production systems here in Canada, according to Dr. Jessie Lavoie, one of the study's senior authors.
Dr. Lavoie says cell and EV therapies require large amounts of high-quality materials for pre-clinical and clinical investigation—and access to low-cost and continuous closed systems is crucial for meeting these requirements.
"Drug innovators in Canada are driven by scientists in academic centers and small biotechnology companies, making it imperative to have efficient and cost-effective solutions," says Dr. Lavoie, an adjunct professor at the uOttawa Faculty of Medicine and a research scientist at Health Canada.
This proof-of-concept study is just the latest work from the uOttawa Faculty of Medicine, establishing new horizons in EVs and immunotherapy research. In recent years, the Faculty's broad research community has emerged as a true innovator in this dynamic area, making new discoveries with potentially broad impact.
Dr. Lisheng Wang, a professor in the Faculty's Department of Biochemistry, Microbiology, and Immunology and the study's other senior author, says the biomanufacturing workflow detailed in the Journal of Extracellular Vesicles could prove to be a game changer down the line since it can potentially offer new options for drug developers exploring cost-effective ways to develop and advance innovative therapies.
Drs Lavoie and Wang say employing hollow-fiber bioreactors is promising for biomanufacturing EVs at scale, yielding far more material than conventional flask-based methods.
When choosing a bioreactor system, the team considered systems that would be affordable for most laboratories, including academic centers, which are big drivers in the development of cell therapy products in Canada. Ultimately, they chose the hollow-fiber bioreactor from Fiber Cell Systems.
More information: Frederic St‐Denis‐Bissonnette et al, A clinically relevant large‐scale biomanufacturing workflow to produce natural killer cells and natural killer cell‐derived extracellular vesicles for cancer immunotherapy, Journal of Extracellular Vesicles (2023). DOI: 10.1002/jev2.12387
Provided by University of Ottawa