This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How a calcium-sensing protein multitasks
The calcium-sensing receptor is critical for maintaining healthy calcium levels, but CaSR is also well-known for its side hustles. The receptor is increasingly recognized for its ability to detect other ions and proteins and for its role in breast cancer, heart disease, diabetes, and other conditions, making it an important drug target for multiple diseases.
But to create CaSR-targeting drugs, designers must first understand how the receptor manages to multitask so that therapies do not impair vital CaSR functions.
So how does CaSR do it all?
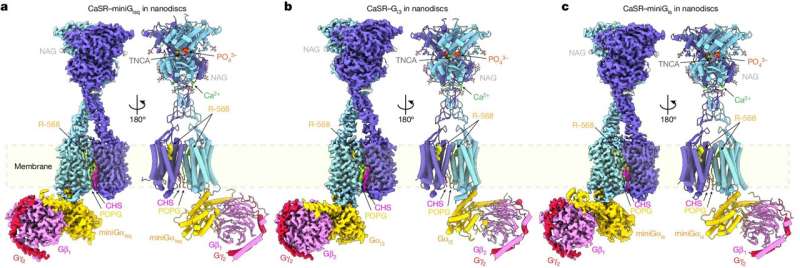
CaSR's ability to multitask and trigger different cellular functions stems partly from its close interactions with a variety of G proteins on the inside of the cell. CaSR can couple to all four different G-proteins, depending on the cell type. In parathyroid cells, CaSR signals through Gq/11 and Gi/o, for example, while in breast tumors it signals through Gs.
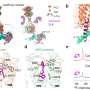
In a new collaborative study conducted by Columbia colleagues Qing Fan and Jonathan Javitch, and Arthur Conigrave at the University of Sydney, the team used cryoEM to visualize CaSR coupling to different G proteins. The study, titled "Promiscuous G-protein activation by the calcium-sensing receptor," was published April 17 in Nature.
Qing Fan, Ph.D., professor of molecular pharmacology & therapeutics, has been uncovering the secrets of CaSR for the past decade using X-ray crystallography and cryoEM to reveal CaSR's shape and gain insights into the way the receptor works. Jonathan Javitch, MD, Ph.D., professor of experimental therapeutics in psychiatry and an expert on cell signaling, provided critical support on the analysis of CaSR function in cells. Arthur Conigrave, MD, Ph.D., professor and endocrinologist at the University of Sydney, has worked with Fan on the CaSR since their initial study in 2016.
Getting such images has been a challenge, partly because it is difficult to assemble a stable complex between CaSR and its various G protein partners, so the study's lead authors, associate research scientists Hao Zuo and Jinseo Park, combined different techniques including the use of miniature, engineered G proteins.
The images helped the team identify elements in both CaSR and G proteins that determine the selectivity of CaSR-G-protein-coupling. A particular loop on the intracellular side of CaSR is indispensable, the team found, and the length and flexibility of this loop allows different types of G protein to bind to CaSR.
"These discoveries may assist the design of therapeutics that target specific CaSR signaling pathways," Fan says. "For example, to prevent broad activation of CaSR throughout the body, targeting the cancer-specific CaSR-induced Gs activation could be considered in breast cancer treatment," Zuo adds.
More information: Hao Zuo et al, Promiscuous G-protein activation by the calcium-sensing receptor, Nature (2024). DOI: 10.1038/s41586-024-07331-1
Journal information: Nature
Provided by Columbia University Irving Medical Center