This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers develop affordable, user-friendly method for single-cell reactions at the nanoliter level
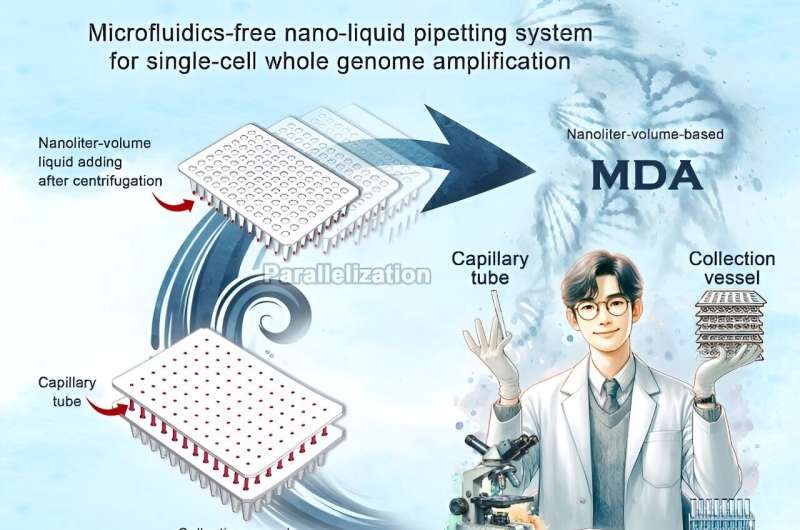
Scaling down single-cell reactions to the nanoliter level is critical to minimize the risk of contamination, increase reaction efficiency, and reduce costs. Researchers from the Single-cell Center of the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences have developed a centrifugally driven system for precise manipulation of nanoliter liquids in single-cell analysis, suitable for conventional biological laboratories.
This system, known as the centrifugally-driven nano-liquid pipetting system (CNPS), provides a low-cost and easy-to-use alternative to microfluidic technology and significantly improves the coverage and uniformity of single-cell genome sequencing.
The results were published in Sensors and Actuators B: Chemical.
"It plays a crucial role in applications such as cell phenotype evaluation, proteomics, genome amplification, and sequencing library construction," said Prof. Li Chunyu, first author of this study from Single-cell Center, highlighting the importance of scaling down reactions to the nanoliter level.
Current methods for constructing a nanoliter reactor for whole-genome multiple displacement amplification from single cells primarily rely on microvalve-based microfluidic devices, microwells, and droplet-based microfluidics.
"These approaches face challenges in the fabrication and operation of microfluidic chips, high equipment costs, and the need for specialized skills," said Li.
In addition, the problem of obtaining nanoliter-volume samples for subsequent analysis, known as the "world-to-chip" interface dilemma, continues to hamper these platforms.
To address these issues, the researchers introduced a breakthrough solution-a low-cost, do-it-yourself CNPS using standard fused silica capillary tubing and a 96/384 PCR plate. "This system uses capillary action for spontaneous sampling and centrifugal force to transfer the measured liquid from the capillary tube to the well plate," said Prof. Ma Bo, co-corresponding author of this study from the Single-cell Center.
Using Saccharomyces cerevisiae, Escherichia coli, and Staphylococcus epidermidis as the molds, the CNPS achieved average coverage of mapped reads of 95.23%, 95.15%, and 80.94%, respectively, which is comparable to droplet microfluidic platforms.
This system has significant advantages over existing nanoliter-volume liquid handling platforms. First, it eliminates the reliance on microfluidic technology, allowing assembly in any biology lab without special training. Second, the closed PCR tube or plate minimizes trace liquid evaporation and reduces contamination. Finally, the seamless transition from the CNPS (nanoliter) to the traditional hand-held pipette (microliter or milliliter) bridges the micro and macro worlds.
In the next phase, researchers will use advanced tools such as Raman-activated single-cell sorting and sequencing and single-cell microdroplet sorting System to automatically isolate single cells in a 96-well PCR plate. They'll then use the CNPS system to efficiently add lysis and DNA amplification reagents in bulk. This process will enable cost-effective parallel amplification of single-cell genomes at the nanoscale.
"The CNPS has promising potential as a robust platform for single-cell analysis, encompassing tasks such as single-cell isolation, cultivation, as well as comprehensive single-cell omic analysis," said Prof. Xu Jian, head of the Single-cell Center.
More information: Chunyu Li et al, A microfluidics-free centrifugally-driven nano-liquid pipetting system (CNPS) for single-cell whole genome amplification, Sensors and Actuators B: Chemical (2024). DOI: 10.1016/j.snb.2024.135391
Provided by Chinese Academy of Sciences