This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Research challenges 'universal mechanism' concept, aiming to understand specific protein interactions
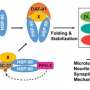
Heat shock proteins (HSPs) are the chaperones of cellular stress response because they help guide the folding and unfolding of other proteins.
Understanding how these chaperones work could also help researchers understand how diseases like Alzheimer's function. While fundamental to the survival and function of cells across the natural kingdoms, HSPs, which are actually just one type of chaperone, can provide a greater understanding of cellular biology as it pertains to stress.
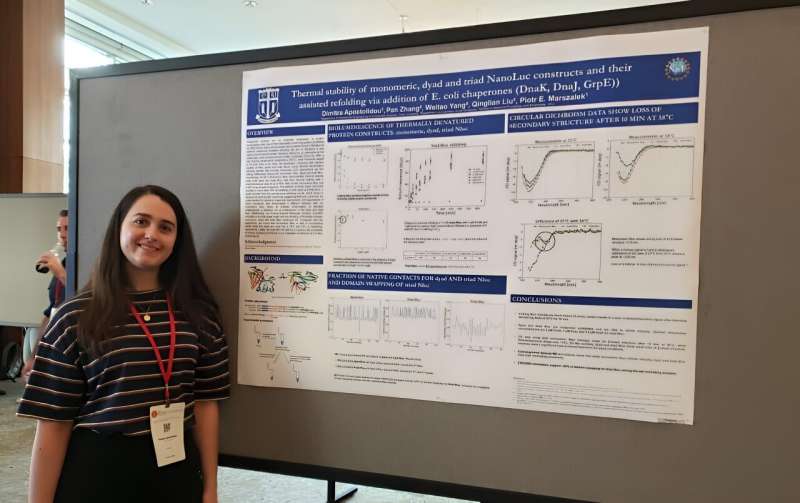
At least that's what Dimitra Apostolidou, a Ph.D. candidate in mechanical engineering and materials science at Duke University, has been researching.
These proteins, which perform their bedrock work from simple organisms like E. coli to complicated ones like humans, offer up insights into the cellular mechanisms that help protect us against stress.
HSPs were initially discovered due to their increased presence in cells exposed to higher temperatures. This discovery highlighted temperature as a critical stress factor, paralleling how fever can detrimentally affect human health. However, Apostolidou points out that HSPs respond not only to thermal stress but also to a myriad of other stress factors, demonstrating their vital role in cellular defense mechanisms.
Apostolidou says the scientific community recognizes five major classes of HSPs, with the HSP70 family being particularly noteworthy. Humans possess up to 17 variations of this protein, contrasting sharply with E. coli, which harbors a single variant known as DnaK.
These proteins perform various functions within cells, including aiding other proteins in maintaining their structure and functionality amidst stressful conditions. She emphasizes the significance of HSPs in regulating vital cellular processes, pointing out how their malfunction can lead to diseases like Alzheimer's, Parkinson's and cataracts due to protein aggregation.
By expanding the range of substrates used in studies, Apostolidou's research aims to deepen our understanding of complex proteins like HSPs and challenge the prevailing idea of a "universal mechanism" at play. "I don't want to come across as though this has solved the problem, but we have to try and understand how these systems work for various proteins," she said.
Substrates are engineered versions of a protein that researchers can use to get a fuller understanding of how chaperones work. Apostolidou's previous work describes a new substrate that could allow new types of studies with individual proteins one at a time.
Her recent work published in Protein Science suggests that not all proteins require the same chaperones for proper function, and though chaperones act upon them, what should really be noted is what the proteins do in response as well as the state they are in. The extent of this highly dynamic relationship could have far-reaching implications for our understanding of cellular mechanics and disease pathology.
Beyond her scientific work, Apostolidou shared that she was driven by a desire to pivot from physics and polymer science to biology, bringing her all the way from Athens to Durham, North Carolina.
"Athens is a big city, but coming to Durham it felt smaller and more intimate," she shared. "One thing I will say, I made many incredible friends through the program—I'd even go grocery shopping with one of them every Sunday."
Though the culture shift was an adjustment for Apostolidou, she credits the research collaborations through labs with Piotr E. Marszalek, professor of mechanical engineering and materials science, and the support of her graduate peers with helping her feel welcome.
"When I joined the lab, the previous graduate students were very welcoming," she said. "After my time in that space, I learned how important collaboration was to everyone there."
More information: Dimitra Apostolidou et al, Tandem repeats of highly bioluminescent NanoLuc are refolded noncanonically by the Hsp70 machinery, Protein Science (2024). DOI: 10.1002/pro.4895
Provided by Duke University