This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Making ends meet: Researchers find that a protein superglue is crucial for DNA damage repair
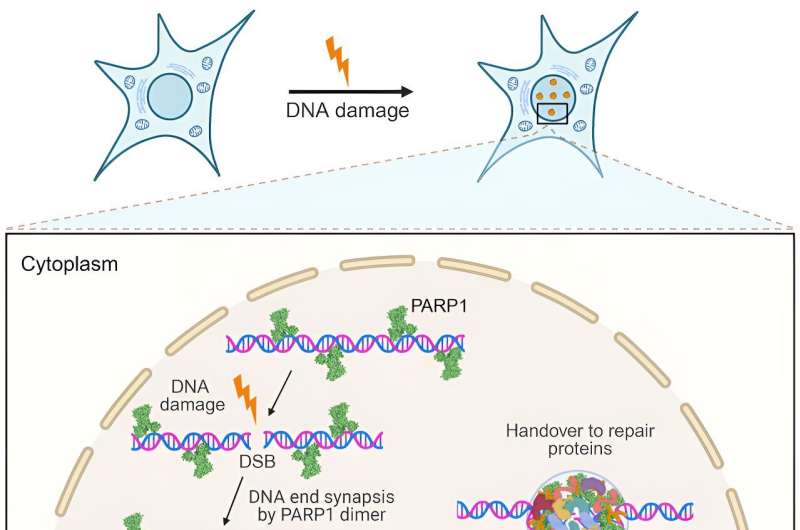
Our DNA undergoes constant damage and repair. The most severe damage happens when the DNA breaks into two pieces, known as a double-strand DNA break. It creates two loose DNA ends that, if left unfixed, can lead to cell death. Researchers from the Biotechnology Center (BIOTEC) of the Dresden University of Technology have now answered the long-standing question of what keeps the broken DNA ends from being separated.
The team discovered that the protein PARP1 becomes an underwater superglue and creates a special healing zone that holds the loose DNA ends together and lets the DNA repair begin. The discovery unveils a key step in DNA damage repair, offering valuable insight for cancer treatment. The results are published in Cell.
"How cells prevent separation of broken DNA ends has been somewhat of a mystery. My team has found that it is thanks to a protein called PARP1 that has long been known to be a sensor of DNA damage," explains Prof. Simon Alberti, research group leader at the Biotechnology Center (BIOTEC) of TUD Dresden University of Technology.
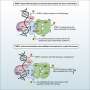
"Individual PARP1 molecules detect the double-strand DNA break and connect with one another to form something that can be regarded as a drop of an underwater superglue that prevents the separation of the two ends. We call this glue a condensate, which is a cluster of tightly interconnected protein and DNA molecules that are isolated from the rest of the cell. This glue forms a special healing zone. It not only keeps the DNA ends together but also lets DNA repair proteins do their job," adds Prof. Alberti.
First responders and a protein cordon
PARP1 is like a first responder at the site of an accident. Its job is to travel along the DNA and patrol it, constantly looking for damaged DNA. Once it locates a double-strand break, it sounds the alarm to call DNA repair proteins that take care of the damage.
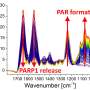
"We could pinpoint the exact molecular events that underlie the formation of DNA damage repair sites, but PARP1 condensation is just the beginning. After it glues together with DNA, PARP1 becomes active as an enzyme and recruits an array of downstream DNA damage proteins," explains Dr. Nagaraja Chappidi, a scientist in the Alberti group who conducted many of the experiments.
PARP1 shields off the DNA damage break from the rest of the environment of the cell nucleus. One can think of it as the first responders identifying the accident site and cordoning off the area. This lets the molecular repairmen do their job in a safe space and fix the broken DNA quickly.
"One of these repair proteins is the protein FUS, which has long been known to be recruited to DNA damage sites, but its function there has remained elusive. We could show that FUS acts like a lubricant and softens the glue so that repair enzymes can come in and do their work," adds Dr. Chappidi.
"It's an example of collective protein behavior that results in higher-order functionality. Every protein does its own job, but they all must collaborate to accomplish the goal of detecting and reversing the DNA damage," says Dr. Titus Franzmann, senior scientist in the Alberti group.
Creating DNA breaks from scratch
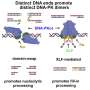
To identify the mechanism, the group used a variety of high-end biochemical and biophysical methods. They teamed up with scientists from the Cluster of Excellence Physics of Life at TU Dresden, the Max Planck Institute of Molecular and Cell Biology in Dresden, the Heinrich Heine University in Düsseldorf, and the Institute of Molecular Biology of the Bulgarian Academy of Sciences.
"We used many different techniques, including single-molecule imaging, optical tweezers, and quantitative biochemistry," explains Dr. Chappidi. "However, the crucial step was recreating the DNA damage scenario in a controllable cell-free system."
Recreating DNA damage sites from the bottom-up in a test tube was central for the study and allowed the team to get unique mechanistic insights into the regulation of DNA repair. "Since it's the first time ever that such a specific DNA damage and repair scenario was recreated outside the cells, our publication provides a detailed protocol so that other groups can take advantage of this new system. We believe it is going to be a great asset for the scientific community that studies DNA damage," adds Dr. Chappidi.
New understanding for cancer treatments
The new study not only shows a step-by-step timeline of what happens after a double-strand DNA break but also provides valuable insights for the cancer research community.
"Because of its role in DNA damage repair, PARP1 is already a target of approved cancer treatments. Inhibiting PARP1 selectively kills off cancer cells. Our work reveals the molecular and physical basis of why these cancer therapies are so successful. Our data suggests a model in which the cancer treatment would impair the PARP1 superglue so that it remains stuck on DNA.
"In this way, it would generate roadblocks for the replication machinery of cancer cells, triggering them to commit suicide. We need more research to confirm the mechanism in more detail," concludes Prof. Alberti.
More information: Nagaraja Chappidi et al, PARP1-DNA co-condensation drives DNA repair site assembly to prevent disjunction of broken DNA ends, Cell (2024). DOI: 10.1016/j.cell.2024.01.015
Journal information: Cell
Provided by Dresden University of Technology