This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research team develops novel high-performance photoelectrode that uses zinc oxide nanopagoda array
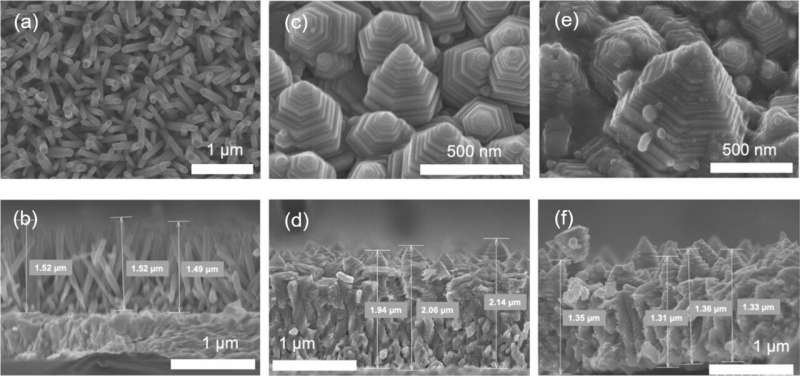
A research team consisting of members of the Egyptian Petroleum Research Institute and the Functional Materials Engineering Laboratory at the Toyohashi University of Technology, has developed a novel high-performance photoelectrode by constructing a zinc oxide nanopagoda array with a unique shape on a transparent electrode and applying silver nanoparticles to its surface.
The zinc oxide nanopagoda is characterized by having many step structures, as it comprises stacks of differently sized hexagonal prisms. In addition, it exhibits very few crystal defects and excellent electron conductivity. By decorating its surface with silver nanoparticles, the zinc oxide nanopagoda array photoelectrode gains visible light absorption properties, enabling it to function under sunlight irradiation.
Photoelectrochemical water splitting using sunlight is expected to be used as a technology to produce clean energy in the form of hydrogen. As key materials for this technology, photoelectrodes must have low overpotential against water splitting reactions, in addition to high solar absorption and charge transfer efficiencies.
For practical applicability, this technology cannot use rare metals as primary materials, and the fabrication process must be industrialized; however, materials that satisfy these requirements have not yet been developed.
Accordingly, the research team solely focused on the zinc oxide nanopagoda array, as such arrays are inexpensive to produce, feature high electron conductivity, and are not vulnerable to raw material depletion. Initially, zinc oxide nanopagoda arrays were considered difficult to fabricate with good reproducibility.
Led by Marwa Abouelela—a third-year doctoral student who is also the lead author of the paper published in Electrochemistry Communications—the team first optimized the synthesis process to ensure high reproducibility. When the photoelectrochemical properties of the obtained photoelectrode were evaluated, a relatively large photocurrent was observed to emerge under pseudo-sunlight irradiation.
In addition to the high charge transfer efficiency associated with low defect density and high surface chemical reaction activity in many steps, an electromagnetic field analysis has revealed that the nanopagoda's unique nanostructure can efficiently capture ultraviolet rays contained in the incident light.
To ensure the effective utilization of visible light, which accounts for 55% of sunlight, the research team further improved the photoelectrochemical properties by decorating the zinc oxide nanopagoda surface with silver nanoparticles that exhibit localized surface plasmon resonance, increasing the photocurrent by approximately 1.5-fold.
The action spectrum of the photocurrent value indicates that this improvement is primarily attributed to the hot electron transfer caused by visible light absorption by the localized surface plasmon resonance of silver nanoparticles. By optimizing the application of silver nanoparticles, it became possible to only improve the photoelectrochemical properties while preventing adverse effects on the properties of the nanopagoda itself.
Associate Professor Go Kawamura, one of the corresponding authors, said, "Zinc oxide nanopagodas were considered for application only to electron gun emitters, utilizing their high charge transfer efficiency. However, because the structure has many steps, our initial idea was that it is highly active against surface chemical reactions and may be suitable for catalyzing photoelectrochemical reactions."
"Having succeeded in fabricating the nanopagoda, we aimed to improve the efficiency of sunlight utilization by applying silver nanoparticles that exhibit localized surface plasmon resonance, and evaluated the effect by electromagnetic field analysis; however, it was found that the zinc oxide nanopagoda captures incident light, especially ultraviolet rays, into its interior. Although this was completely unexpected, it was a fortunate discovery, as this property contributes to the improvement of photoelectrochemical properties."
Future outlook
Currently, Marwa and students of the same laboratory are leading an investigation into the effect of precise structural control of zinc oxide nanopagodas, as well as surface decoration with other materials, on the photoelectrochemical properties of said pagodas. Because zinc oxide is prone to photocorrosion, it cannot withstand long-term sunlight irradiation by itself, leading us to focus on improving durability via surface decoration.
Upon achieving both high photoelectrochemical properties and durability, the team plans to carry out water splitting hydrogen production in a real environment (decomposition of river water or seawater by sunlight).
More information: Marwa Mohamed Abouelela et al, Ag nanoparticles decorated ZnO nanopagodas for Photoelectrochemical application, Electrochemistry Communications (2023). DOI: 10.1016/j.elecom.2023.107645
Journal information: Electrochemistry Communications
Provided by Toyohashi University of Technology